
Credit: Mark Hoyer, UF/IFAS
Aquatic Plants in Florida Lakes
Information Circular 111
UF/IFAS Florida LAKEWATCH
UF/IFAS School of Forest, Fisheries, and Geomatics Sciences, Program in Fisheries and Aquatic Sciences, Gainesville, Florida
October 2007
A Beginner's Guide to Water Management
Aquatic Plants in Florida Lakes Information Circular 111
This publication was produced by UF/IFAS Florida LAKEWATCH
University of Florida/Institute of Food and Agricultural Sciences
UF/IFAS School of Forest, Fisheries, and Geomatics Sciences, Program in Fisheries and Aquatic Sciences
7922 NW 71st Street Gainesville, FL 32653-3071
Phone: (352) 392-4817
Toll Free 1-800-525-3928
Fax: (352) 392-4902
E-mail: fl-lakewatch@ufl.edu
https://lakewatch.ifas.ufl.edu/
Copies are available for download from the Florida LAKEWATCH website: https://lakewatch.ifas.ufl.edu/ or from the UF/IFAS Electronic Document Information Source (EDIS) website: https://edis.ifas.ufl.edu
Limited reproduction of and/or quotation from this circular is permitted, providing proper credit is given.
Previous editions of this guide were edited and designed by Allison Slavick, ww
Excellent editorial changes were added to this edition by Mike D. Netherland, USACE ERDC Environmental Laboratory, Center for Aquatic and Invasive Plants
The following is a list of all Beginner's Guides to Water Management. We encourage you to read those that pertain to your individual lake-management needs:
- The ABCs: Descriptions of commonly used terms. Information Circular 101 (https://edis.ifas.ufl.edu/fa078). 2013.
- Nutrients. Information Circular 102 (https://edis.ifas.ufl.edu/fa079). 2013.
- Water clarity. Information Circular 103 (https://edis.ifas.ufl.edu/fa080). 2013.
- Lake Morphology. Information Circular 104 (https://edis.ifas.ufl.edu/fa081). 2013.
- Symbols, Abbreviations & Conversion Factors. Information Circular 105 (https://edis.ifas.ufl.edu/fa102). 2013.
- Bacteria. Information Circular 106 (https://edis.ifas.ufl.edu/fa103). 2013.
- Fish Kills. Information Circular 107 (https://edis.ifas.ufl.edu/fa104). 2013.
- Color. Information Circular 108 (https://edis.ifas.ufl.edu/fa105). 2013
- Oxygen and Temperature. Information Circular 109 (https://edis.ifas.ufl.edu/fa106). 2013
- Fish Communities and Trophic State in Florida Lakes. Information Circular 110 (https://edis.ifas.ufl.edu/fa162). 2013.
As always, we welcome your questions and comments.
First published 2007; revised and adapted for EDIS February 2017.
Abstract
This circular represents a summary of current knowledge on aquatic plants and aquatic plant management strategies, highlighting the Florida situation. The major focus of this circular is the management of aquatic plants as opposed to dealing with nutrients, algae, or water clarity. Included are sections on 1) Aquatic Plant Biology, 2) Aquatic Plant Management Problems, and 3) Aquatic Plant Management Techniques.
Note: Circular 111 is available in Portable Document Format (pdf) only. It can be obtained as a single PDF file by clicking on the "Printer Friendly Version" link below.
https://lakewatch.ifas.ufl.edu/media/lakewatchifasufledu/extension/circulars/111AquaticPlants.pdf
Table of Contents
Acknowledgements p. 2
Preface p. 2
Introduction and Overview p. 3
Section 1: Essentials of Aquatic Plant Biology p. 4
- Introduction
- Types of Aquatic Plants
- Littoral Zone
- Limnological and Physical Factors that Determine Plant Distribution and/or Abundance
- Light Availability
- Trophic State, Plant Nutrition, and Water Chemistry
- Substrate Characteristics
- Lake Morphology—An Integrating Factor
- Wind Energy and Watershed Characteristics
- The Influence Aquatic Plants have on Limnology of the Littoral Zone
- Physical and Chemical Components
- The Biotic Component
Section 2: Aquatic Plant Management Problems p. 19
- Introduction
- Visible Problems
- Invisible Problems
Section 3: Aquatic Plant Management Techniques p. 28
- Introduction
- Physical Removal
- Habitat Alteration
- Biological Control
- Herbicides
- Environmental Considerations
- Fate of Aquatic Herbicides in the Environment
- Maintenance Control of Aquatic Weeds
- Manipulating Plant Communities
Conclusion p. 45
Literature Cited p. 47
Acknowledgements
The Florida LAKEWATCH program funded this circular. We thank the many Florida citizen scientists that make Florida LAKEWATCH one of the most successful volunteer monitoring programs in the country if not the world. We also thank Michael D. Netherland, USACE ERDC Environmental Laboratory/Center for Aquatic and Invasive Plants, University of Florida, for writing sections of this circular.
Preface
This circular has been prepared by Florida LAKEWATCH, UF/IFAS Program in Fisheries and Aquatic Sciences, School of Forest Resources and Conservation, and the Center for Aquatic and Invasive Plants of the University of Florida. Much of the material for this circular has been taken and modified from the Aquatic Plant Management in Lakes and Reservoirs manual, which was produced by the North American Lake Management Society (PO Box 5443, Madison, WI 53705- 5443) and the Aquatic Plant Management Society (PO Box 1477, Lehigh, FL 33970).
Information Circular #111 represents a summary of existing knowledge on aquatic plants and aquatic plant management strategies, with a focus on the situation in the Florida. The major focus of this circular is the management of aquatic plants as opposed to dealing with nutrients, algae, or water clarity. Readers will find practical information on those subjects and general water management information for Florida lakes in Florida LAKEWATCH Circulars #101 (A Beginner's Guide to Water Management—The ABCs at https://edis.ifas.ufl.edu/fa078), #102 (A Beginner's Guide to Water Management—Nutrients at https://edis.ifas.ufl.edu/fa079), and #103 (A Beginner's Guide to Water Management—Water Clarity at https://edis.ifas.ufl.edu/fa080). The science of aquatic plant management, like that of lake management, continues to evolve. New information will emerge over time. Readers are therefore urged to consult knowledgeable professionals for information on recent advances in the field of aquatic plant management.
Finally, the North American Lake Management Society (NALMS) and the Aquatic Plant Management Society (APMS) recognize that citizens often hesitate to tread on the territory staked out and vigorously defended by "experts." NALMS and APMS, however, encourage private citizens to take an active part in developing comprehensive lake management plans that include aquatic plant management. NALMS and APMS also urge professionals to work with citizens. Although working with a diverse group of nonprofessionals may be frustrating, experts by themselves cannot manage lakes. Florida LAKEWATCH, the Department of Fisheries and Aquatic Sciences, and the Center for Aquatic and Invasive Plants agree wholeheartedly. As many citizens of Florida as possible should be part of the solution.
Introduction and Overview
Control the weeds! This simple and eminently reasonable-sounding management guideline causes more lake-related controversy than possibly any other. Once someone mentions that a Florida lake looks a little weedy, controversy invariably follows. Quarrels typically break out between and among user-groups, scientists, and management/regulatory agencies over whether the plants in question are weeds; if they're weeds, whether they're a problem; and if they're a problem, whether they're a problem that must be managed. In the event that an agreement is finally reached that the weeds must be dealt with, quarrels then tend to erupt over how much of the aquatic vegetation should be controlled. If the desirable level of vegetation management can be established, still more quarrels then develop over how to achieve those levels. Should nutrient control be instituted? Should aquatic herbicides be used or should mechanical harvesting be used? Should biological controls like grass carp be used? Should a combination of management techniques be used?
Faced with what seem to be unending questions and controversies, many Floridians and some government agencies often choose the "Do Nothing" or "Delay" option. In rare cases, doing nothing or delaying a decision has turned out to be the best course of action to manage an aquatic weed problem, but the history of aquatic plant management in Florida has shown that delay and inaction are frequently chosen at the wrong time or for the wrong reasons and that an unmanaged problem usually becomes bigger and harder to solve. When nothing is done to manage them or their management is delayed, the abundance of aquatic plants in Florida's waters can reach truly problematic levels. Ignored for long enough, small problems tend to become noticeable—and at that point they are frequently declared emergencies. Efforts to make a weed problem go away quickly usually create more—and much worse—problems. It is therefore nearly always best to act as soon as you detect an aquatic weed problem in a lake you manage. Better yet, have a plan in place before a problem develops.
A well-evaluated and carefully designed management plan must be developed for each water body. A management plan that addresses aquatic plants and that the primary stakeholders have agreed to in advance will eliminate controversy and management delays if a problem should arise. With reasonable care in the decision making process, aquatic plants can be managed successfully without destroying the desirable attributes of lakes that attract us to these water bodies.
Many of the conflicts that arise over the management of aquatic plants in lakes are rooted in differences in educational background, philosophy, experience, and even differing perspectives based on what region of the country our citizens may have come from. This circular is written to provide the citizens of Florida and visitors to our state a better understanding of why aquatic plants are managed as they are. Besides providing information on the concepts and techniques of aquatic plant management, the role of aquatic plants in Florida's lakes is also discussed.
The focus of this circular is the management of aquatic macrophytes, lake plants large enough to be observed by the naked eye. This diverse group of aquatic and wetland plants includes flowering vascular plants, mosses, ferns, and macroalgae. This publication emphasizes the management of aquatic plants in lakes, but much of the information in it should also be useful to anyone who manages aquatic plants in reservoirs, ponds, and flowing-water systems such as canals and rivers. This circular provides information on the majority of aquatic plant management options currently available for large-scale use and previews a few experimental techniques that may be used in the future. Most importantly, the pros and cons of using different techniques are discussed along with the potential trade-offs among alternative options given different lake uses. The information in the circular is the best available on aquatic plant management. The professionals of UF/IFAS Florida LAKEWATCH and researchers at the UF/IFAS Program in Fisheries and Aquatic Sciences School of Forest Resources and Conservation and the Center for Aquatic and Invasive Plants have contributed to this circular and rely on it, themselves.
Overview
Section 1, Essentials of Aquatic Plant Biology, describes how aquatic plants fit into the ecology of Florida lakes. Understanding the role of aquatic macrophytes in water bodies, especially with regard to water quality and fisheries, is critical to the development of sound management plans. All readers are strongly urged to read Section 1 completely because this section reveals many relationships between aquatic plants and lake ecology that should be understood before developing an aquatic plant management plan.
Section 2 addresses the question of whether there is a weed problem at a lake. This section focuses on how to define the problem and identify possible causes for the problem.
Section 3 discusses the various aquatic plant management techniques that are currently available for managing nuisance growth of aquatic weeds. Specific attention is given to mechanical, chemical, and biological controls with discussion of the pros and cons of using these techniques.
Section 1: Essentials of Aquatic Plant Biology
Introduction
Much aquatic plant research has been stimulated by the need to control nuisance species such as hydrilla (Hydrilla verticillata), water hyacinth (Eichhornia crassipes), Eurasian watermilfoil (Myriophyllum spicatum), elodea (Elodea canadensis), coontail (Ceratophyllum demersum), and alligator-weed (Alternanthera philoxeroides). Understanding aquatic plant biology is important to the immediate problems of managing aquatic plants and aquatic ecosystems, and it makes the development of new management techniques, the application of present techniques, and the assessment of environmental impacts more efficient. Interest is growing in restoring and restructuring macrophyte communities and there is a new appreciation for the littoral zone (the area of a lake that extends from the shoreline to the greatest depth occupied by rooted plants). There is also a need to make management results more predictable, especially when considered in a long-term ecosystem context.
The development of effective and environmentally acceptable aquatic plant management programs also requires some knowledge of lake limnology. Limnology is the scientific study of the physical, chemical, geological, and biological factors that affect aquatic productivity and water chemistry in freshwater ecosystems—lakes, reservoirs, rivers, and streams. Many limnological processes affect the species, distribution, and/or abundance of aquatic plants that will be present in a water body. Making things more complicated, aquatic plants can also impact limnological processes like nutrient, chemical and temperature regimes and other biota in a lake or reservoir, especially in the littoral zone.
A single circular cannot review all the aquatic plant biology and limnology that might be relevant to aquatic plant ecology, but interested managers may explore several good technical textbooks that go into great detail on the ecology of aquatic plants (Hutchinson 1975; Wetzel and Hough 1983; Cole 1983) and the biology and control of aquatic plants (Gettys, Haller, and Bellund 2009). This circular focuses on what will be most useful to aquatic plant management efforts and includes information about
- types of aquatic plants,
- the littoral zone,
- the limnological and physical factors that determine plant distribution and abundance,
- the influence that aquatic plants have on the limnology of the littoral zone, and
- the biotic component: relationships between aquatic plants and other organisms including epiphytes, macroinvertebrates, fish, and wildlife.
Types of Aquatic Plants

Credit: Vic Ramey, UF/IFAS Extension
The types of aquatic and wetland plants (macrophytes) of interest to most aquatic plant management programs can be classified into four groups: emergent, floating-leaved, submersed, and free-floating. Aquatic macrophytes are the macroscopic (large enough to be observed by the naked eye) forms of aquatic and wetland plants found in water bodies, including flowering vascular plants, mosses, ferns, and macroalgae.
Emergent macrophytes are plants that are rooted in the lake bottom with their base portions submersed in the water and their tops extending into the air. They grow on periodically inundated or submersed soils. Most emergent macrophytes are perennials, which means that entire plants or part of plants live for longer than one year. The habit of emergent macrophytes to root under the water in the substrate and leaf and flower in the air is ideal for plant growth. Nutrients are available from the sediment, water is available from both the sediment and the overlying water, and carbon dioxide and sunlight are available to the emergent portions of the plant.
Emergent plants have to be strongly rooted; much of their energy is devoted to producing a strong structure to withstand the wind and waves in the shallow water zone. Many plant species need mud flats for their seeds to germinate, but they can spread into deeper water by sprouting from rhizomes, which are expanding roots or underground stem systems. In northern climates, the dry, dead stems often supply oxygen for root respiration during the winter, when the lakes are covered with ice. Cutting off dead stems below the water surface before the lake freezes limits oxygen supplies and sometime kills the rhizomes—a potentially effective management technique in northern cold climates, but one that is not much use in Florida.

Credit: Vic Ramey, UF/IFAS Extension
Common emergent macrophytes include plants such as bulrushes (Scirpus spp.), cattails (Typha spp.), reeds (Phragmites spp.), spikerushes (Eleocharis spp.) maidencane (Panicum hemitomon), pickerelweed (Pontederia cordata), and duck potato (Sagittaria lancifolia). Some emergents, wild rice (Zizania spp.), for example, develop submersed or floating leaves before mature aerial leaves form.
Floating-leaved macrophytes (plants that are rooted to the lake bottom, with leaves that float on the surface of the water) generally occur in areas of a lake that always remain wet. Common representatives include waterlilies (Nymphaea spp.), spatterdock (Nuphar spp.), and watershield (Brasenia spp.). Floating leaves are attached to roots or rhizomes with a flexible, tough stem (actually in many cases a leaf stalk). Some floating-leaved macrophytes, like Nuphar spp., can exist in a submersed form for a considerable time. Many floating-leaved species form large colonies from spreading underground rhizomes. In northern climates, under winter low-water conditions, frost will often "heave" the rhizomes up out of the lake bottom, which helps thin dense stands.
Floating-leaved plants live in two extremely different habitats: the bottom of the plant lives in the water, and the top of the plant lives in air. A thick, waxy coating covers the top of the leaf to keep it from drying out in the air. The waxy coating makes herbicidal control of this plant type difficult because it repels herbicides. Herbicides are more effective against floating-leaved plats if they are mixed with special chemicals called adjuvants that act as wetting agents and help the herbicide stick to and penetrate the waxy surface. Adjuvants are also used on many kinds of emergent and free-floating species when treating with herbicides because these plants also have protective coatings. The waxy coating also tends to be present on most emergent aquatic plants and not specific to floating leaved species.
Submersed macrophytes (plants that grow completely under the water) are a diverse group that includes quillworts (Isoetes spp.), mosses (Fontinalis spp.), muskgrasses (Chara spp.), stoneworts (Nitella spp.), and numerous vascular plants. Many submersed plants, such as widgeon-grass (Ruppia maritima), various pondweeds (Potamogeton spp.), and tape-grass (Vallisneria spp.), are native to the United States. Others like hydrilla are exotic and cause some of the worst aquatic weed problems. These invasive plants tend to grow rapidly to the water surface, and they can form dense canopies in the upper water column that interfere with both the use and the aesthetics of the water body.

Credit: Mark Hoyer, UF/IFAS Extension
Submersed species face special problems. Under the water, light for photosynthesis and carbon dioxide for respiration are in short supply. However, submersed species have lower needs for these because they are supported by the water and therefore do not need to devote very much energy to structural support. Water supports about 95% of the weight of this type of plant.
Free-floating macrophytes (plants that typically float on or just under the water surface with their roots in the water and not in the sediment) are also a diverse group of aquatic plants. Small free-floating plants include duckweeds (Lemna spp.), mosquito fern (Azolla caroliniana), water meal (Wolffia columbiana), and water fern (Salvinia spp.). Larger free-floating plants, including water hyacinth and water lettuce (Pistia stratiotes), are the number one targets for aquatic plant management in Florida.
Free-floating species are entirely dependent on the water for their nutrient supply. In fact, some (e.g., water hyacinth) have been used in wastewater treatment to remove excess nutrients. If nutrient limitation will work for macrophyte management, this is the group for which it will most likely work. Free-floating plants are also the only aquatic plants not constrained by water depth. The location of these plants is at the whim of wind, waves, and current, so they will likely be found in quiet locations and embayments. Free-floating plants grow and multiply extremely quickly. For example, water hyacinth plants can double in ten days and 10 plants can become almost 41,000 plants in 120 days (Figure 6). For this reason, water hyacinths can cover nearly the entire surface of ponds, lakes and rivers (not just quiet locations and embayments).
The above are general descriptions of aquatic plant groups and some of the biology pertinent to their management. One excellent resource for this type of information is the Aquatic Plant Information Retrieval System at the UF/IFAS Center for Aquatic and Invasive Plants Plants, 7922 NW 71st Street, Gainesville, FL 32653 https://plants.ifas.ufl.edu/). Control tactics are often species-specific, which means that to develop effective management plans, you will need to know exactly what species are present, where they are located, and in what abundance that they occur. This takes some technical knowledge, but help is usually readily available through natural resource agencies, universities, museums, natural history surveys, and private consultants.

Credit: Vic Ramey, UF/IFAS Extension
Littoral Zone
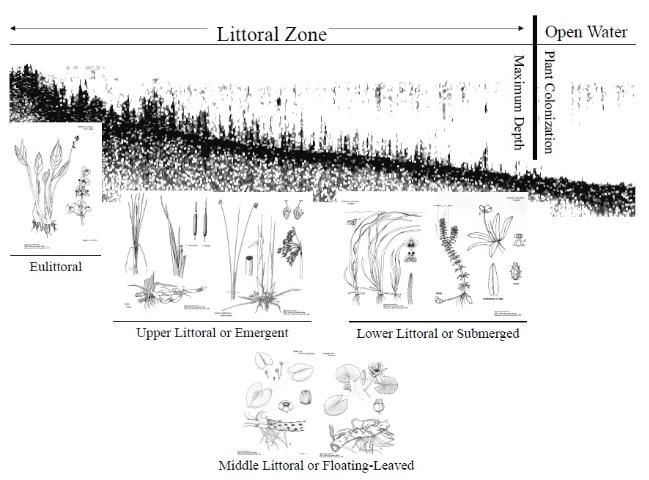
Rooted aquatic plants inhabit the littoral zone, the interface between dry land and open water of lakes and reservoirs. The littoral zone is defined by where rooted plants will grow (Figure 7). It is the area from the lake's edge to the maximum water depth where rooted plant growth occurs. Because most lakes and reservoirs in the United States and especially Florida are relatively small and shallow, the littoral zone often contributes significantly to a water body's productivity, and it can be a major factor regulating lake or reservoir ecosystems. The littoral zone has traditionally been divided into four rather distinct transitional zones: the eulittoral, upper littoral, middle littoral, and lower littoral.
Credit: UF/IFAS Extension
The eulittoral zone constitutes that part of the shoreline that lies between the highest and lowest seasonal water levels and often contains many wetland plants. The upper littoral zone is commonly called the emergent plant zone and is generally dominated by emergent plants. This zone extends from the waters edge to depths of about 3 to 6 feet (1 to 2 m). The middle littoral zone is deeper and is generally dominated by floating-leaved plants like fragrant waterlily (Nymphaea odorata), yellow waterlily (Nymphaea mexicana) and American lotus (Nelumbo lutea). The middle littoral zone extends lakeward from the upper littoral zone to water depths of 3 to 9 feet (1 to 3 meters). Finally, the lower littoral zone is the deepest zone where most submersed plants are found and typically extends from the floating-leaved plant zone down to the limits of the photic zone (the area of a lake where photosynthesis can occur, defined by the depth to which at least 1 percent of the surface light intensity penetrates). The depth of the photic zone is dependent on water clarity, which is primarily determined by the amount of algae in the water (see Florida LAKEWATCH Circular #103, A Beginner's Guide to Water Management— Water Clarity).
Limnological and Physical Factors that Determine Plant Distribution and/or Abundance
Different species of aquatic plants live in different "worlds," with sediment, water, and air in different combinations. Most aquatic plants are secondarily adapted to live in the water, having once lived on land. They gradually evolved the mechanisms that permit them to live in a watery world. The most important environmental factors affecting the abundance and distribution of aquatic macrophytes in lakes include light availability, lake trophic state (nutrient richness) characteristics and their effects on water chemistry, sediment characteristics, wind energy, lake morphology (the surface area, shape, and depth of the lake), and watershed characteristics. All of these factors can work independently or in combination to determine the distribution and abundance of aquatic plants in lakes.

Credit: UF/IFAS Extension
Light Availability
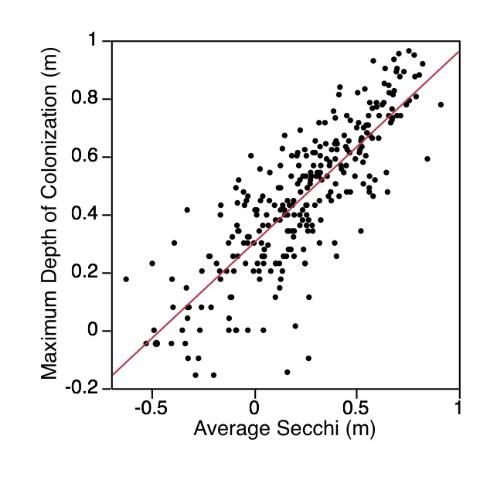
Aquatic plants require light for growth, thus light availability is often considered the single most crucial environmental factor regulating the distribution and abundance of aquatic plants. Light availability is directly linked to water clarity, and, as water depth increases or water clarity decreases, both the amount and the quality of light for photosynthesis at the lake bottom diminishes. Generally, submersed macrophytes will grow to a depth where at least 10% of the ambient surface light is available. This depth can be estimated by multiplying the Secchi depth (depth at which a black and white disk lowered into a lake disappears) by 1.7. If the majority of a lake's bottom exceeds 1.7 times the Secchi depth, the lake will have fewer aquatic macrophytes. Even shallow lakes, if they are turbid enough, will have sparse aquatic plant growth on the bottom.
Recent work by Florida LAKEWATCH graduate students and staff has defined the relationship between maximum depth of aquatic plant colonization and Secchi depth for 279 Florida lakes (Figure 8), (Caffrey, Hoyer, and Canfield 2007). LAKEWATCH data showing the relationship between plant colonization and Secchi depth was similar to data from other parts of the country and world, suggesting that this relationship is robust and can be used to help determine aquatic plant management strategies for lakes. In fact, Florida LAKEWATCH staff (Hoyer et al. 2005) successfully used published relationships between maximum depth of aquatic plant colonization and Secchi depth to estimate changes in the potential aquatic plant coverage of some Florida lakes that have large fluctuations in water level.
Florida LAKEWATCH Circular #103 (A Beginner's Guide to Water Management—Water Clarity) describes factors that determine water clarity in lakes. Briefly, water clarity is determined by the abundance of phytoplankton, organic color, and both organic and inorganic suspended particles present in the water. Lakes with low phytoplankton concentrations and low color values have high water clarity. As phytoplankton and color levels increase, there is a rapid reduction in water clarity, aquatic macrophytes become light-limited, and the size of the littoral zone decreases. Conversely, the size of the littoral zone can increase if phytoplankton or color levels decrease. Non-algal suspended particle (suspended solid) concentrations in lakes are determined by the continuous processes of surface runoff input, loss to sedimentation, and re-suspension of the bottom. Shallow lakes that are open to the wind and that have substantial layers of soft sediments often have high suspended solid concentrations because wind mixes their bottom sediments. Suspended solids limit light for plant growth and decrease littoral zone size. Boat traffic, shoreline erosion, and biotic factors such as fish (e.g., the common carp or catfish) feeding on the bottom can also increase suspended sediment.
Trophic State, Plant Nutrition, and Water Chemistry
All things being equal, nutrient-poor lakes are less productive than nutrient-rich lakes. The primary factor determining the trophic state of a lake (i.e., its nutrient richness) is the geologic region where the lake occurs. Soils are determined by the surrounding geology, and some simply have more nutrients than others. Additionally, watershed management practices and human-caused nutrient additions can also be important in determining nutrient levels in lakes. These nutrients in turn generally result in more algal growth, which decreases water clarity and thus decreases available light for aquatic plants (see Florida LAKEWATCH Circular #102, A Beginner's Guide to Water Management—Nutrients).
Some lake managers believe that nutrients can limit the growth of aquatic plants. However, there are few substantiated reports of nutrient-related growth limitation for aquatic plants. Nutrients supplied from sediments, combined with those in solution, are generally adequate to meet nutritional demands of rooted aquatic plants, even in oligotrophic (nutrient-poor) systems. While this information suggests that nutrients do not limit growth of aquatic plants in oligotrophic lakes, a large survey of Florida lakes (Canfield and Hoyer 1992; Hoyer et al. 1996) indicated that these lakes generally do maintain less total biomass of aquatic plants and usually different species than eutrophic (nutrient-rich) lakes. Even though this is true for extremes on the nutrient continuum, nutrient control is probably not a viable tool for aquatic plant control in lakes.
Rooted macrophytes usually fulfill their phosphorus (P) and nitrogen (N) requirements by direct uptake from sediments. The role of sediments as a direct source of P and N for submersed macrophytes is ecologically quite significant because available forms of these elements are normally in very low concentrations in the open water of most aquatic systems, especially during the growing season. Likewise, the availability of micronutrients in the open water is usually very low, but they are relatively available in most lake sediments. However, the preferred source of some required nutrients such as potassium (K), calcium (Ca), magnesium (Mg), sulfate (SO4), sodium (Na), and chlorine (Cl) appears to be the open water. Submersed macrophytes use nutrients from both the water and the sediment. Whether they take up nutrients through their roots or from their shoots and leaves depends on the availability of nutrients in the sediment compared to the water. In other words, submersed plants are opportunistic species that get nutrients from the most easily available source.
Inorganic carbon is the nutrient most likely limiting photosynthesis and growth of submersed macrophytes. The difficulty plants have in capturing carbon dioxide (CO2) and transporting it throughout the plant is known to limit photosynthesis in terrestrial plants. This aspect is even more critical in submersed aquatic plants because the diffusion of CO2 into lake water is slow. The free CO2 dissolved in water is the most readily used carbon form by freshwater submersed plants for photosynthesis. Some species of aquatic plants can use bicarbonate (HCO3) as a carbon source, but the process by which they use it is inefficient. The ability to use bicarbonate is an adaptive advantage for these plants because in many freshwater systems the largest fraction of inorganic carbon exists as bicarbonate.
Besides influencing growth, general water chemistry (i.e., pH, alkalinity, specific conductance) influences the species composition in lakes and is an important factor determining plant distribution over broad geographic regions. For example, Hoyer et al. 1996 found that water-moss (Fontinalis spp.) occurred in 32 of 322 lakes that had an average pH of 5.2, while bacopa (Bacopa monnieri) occurred in 57 of the 322 lakes that had an average pH of 7.4. Apparently, these two plant species need different water chemistries to survive. There are large water chemistry gradients in the waters of the world including; hardwater/softwater, acid/alkaline, oligotrophic/eutrophic —but usually there are some plant types than can live in any combination of chemistries.
Substrate Characteristics
Bottom sediments act as a nutrient source and anchoring point for aquatic plants. Not all lake bottoms can support plant life. Rock or cobble lake bottoms are so hard that plant roots cannot penetrate them, whereas muck lake bottoms are so soft that plant roots can't anchor in them. Flocculent, or loose and woolly sediments, which are commonly called "muck" bottoms, are too unstable to hold plants securely. Substrates that fall somewhere along the continuum between rock and flocculent organics will generally support aquatic plants if they receive sufficient nutrients and light.
Another substrate factor that may limit the growth of aquatic plants is anaerobic (devoid of oxygen) conditions. Low dissolved oxygen concentrations in sediments can cause a host of chemical conditions that can be toxic to aquatic plants. High concentrations of soluble reduced iron, manganese, and sulfides including S=, HS-, and H2S are highly toxic to plants. High soluble-iron concentrations interfere with sulfur metabolism and limit the availability of phosphorus. Sediments containing excessive organic matter often contain high concentrations of organic acids, methane, ethylene, phenols, and alcohols that can be toxic to some types of vegetation. The above conditions are most frequently found in anaerobic sediments of eutrophic or hypereutrophic lakes. Some plants are specialists at dealing with these types of conditions. Aquatic plants can protect themselves from these toxins to some degree with oxygen released from roots, which eliminates the anaerobic conditions that create the toxic substances.
Lake Morphology—An Integrating Factor
Water clarity, trophic state, water chemistry, substrate type, and the actions of the wind and waves determine aquatic plant distribution and abundance. These parameters are interrelated and interact with the lake's basin depth, bottom slope, surface area, and shape to determine littoral zone size (aka, lake morphology). For a good overall description of lake morphology see LAKEWATCH Circular #104 A Beginner's Guide to Water Management—Lake Morphology.
Lake basin forms are extremely variable and reflect the water body's mode of origin. Lake basins are continuously modified with water movements and sediment inputs from the basin's watershed. As the form of a lake basin changes, the size of the littoral zone in relation to a lake's open water changes. Most water bodies become shallower as they age. Unless something or someone intervenes, littoral zone size increases as a water body gets older.
Water depth is one of the most critical environmental factors determining the lakeward extent of the littoral zone and the type of plants that grow in a water body. Where a lake's substrate exceeds approximately 1.7 times the Secchi depth, submersed aquatic plants will be light limited and generally unable to grow. With some exceptions, a depth range between 30 and 45 ft (9 and 14 m) is the limit for most aquatic plants, even if light is available. Emergent and floating-leaved plants seldom grow in water exceeding 10 ft (3 m), so deep lakes also have limited emergent communities.
The steepness of the littoral slope is inversely related to the maximum biomass of submersed macrophytes, which is probably due to the difference in sediment stability on gentle and steep slopes. A gently sloping littoral zone allows the deposition of fine sediments that promote plant growth. Steeply sloped littoral zones are areas of erosion. Unstable, moving sediment will not support plant growth. The manipulation of lake depth and slope are both powerful tools when encouraging or discouraging the growth of aquatic plants in specific areas of a lake.
Wind Energy and Watershed Characteristics
See UF/IFAS Florida LAKEWATCH Circular #104, A Beginners Guide to Water Management—Lake Morphometry at https://edis.ifas.ufl.edu/fa081
All lakes have a shoreline-water interface that receives energy from wind and waves. Surface area and shape significantly influence the effect wind can have on wave size and current strength. Large lakes tend to have larger fetches (the fetch is the area of the lake that is open to the prevailing wind) and thus have greater wave and current energy than lakes with small surface areas. Wave action and currents erode a terrace along the shoreline, leaving coarse material in shallow water and depositing finer materials in deep water. The direction and strength of the wind and the slope and shape of the lake basin determine where the substrates will move. Generally, points and shallows where wind and wave energy are highest tend to be swept clean. Bays and deep spots in a lake tend to fill with sediment. In England, Pearsall (1920) demonstrated that the variation in the quantity and quality of silt largely controls the distribution of submersed vegetation. Large lakes with many bays or coves may develop an extensive littoral zone because these areas are protected from strong waves and currents. Thus, basin size, shape, and depth determine to a large degree the distribution of sediments in a lake and therefore the distribution of aquatic plants.
The Influence Aquatic Plants have on Limnology of the Littoral Zone
Up to this point, we discussed the effects of the environment on aquatic plants. Now, it is time to discuss the converse—the effects that aquatic plants have on their environment. Natural ecosystems can experience massive changes in aquatic plant biomass over time scales of decades to centuries. Management practices and the introduction of new species produce equally large changes over time scales of weeks or months. These changes in the species composition, distribution, and abundance of aquatic plants impact lake ecosystems by altering physical, chemical, and biological aspects of the littoral zone and potentially whole lake systems.
The following relationships are complicated and describing them in detail is beyond the scope of this circular. We offer the following general descriptions to let the reader know that managing or not managing the aquatic plants in a lake can cause rippling effects throughout the lake system.
Physical and Chemical Components
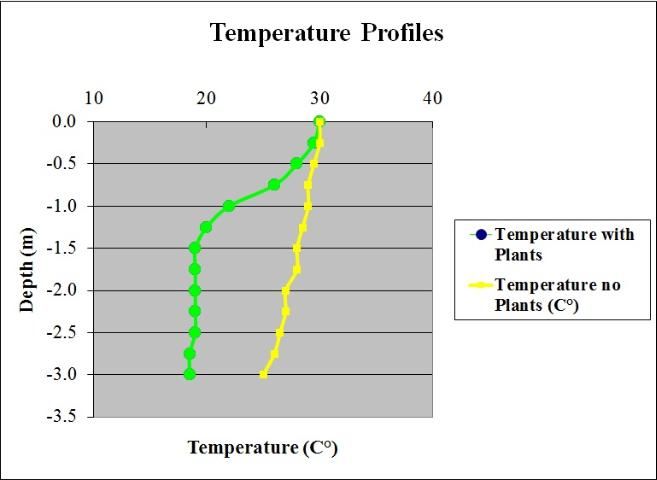
Dense stands of aquatic plants form a heavy shading canopy that reduces the light under the plants. This shading along with reduced water circulation in dense plant stands allows the formation of vertical temperature gradients as steep as 18°F (10°C) over 3 ft (1m) of water (Figure 4). Because water circulation is reduced in plant beds, sediment that would otherwise be suspended in the water column sinks instead and builds up on the lake bottom. Aquatic plant beds also act as a sieve, retaining coarse particulate organic matter that enters the lake from storm water. Aquatic plants and attached algae (epiphytic algae) themselves produce tremendous amounts of organic matter through photosynthesis. The organic matter produced then falls to the bottom, further building the lake floor. All of these mechanisms tend to increase the accumulation of sediments on the lake floor, which is usually undesirable for people who use these areas of a lake. Over the short term, organic matter accumulation creates a food source for benthic (bottom dwelling) organisms. However, over the long term, accumulation of organic sediments causes expansion of the littoral zone and filling in of the lake. In general, macrophyte stands are sinks for particulate matter and sources of dissolved phosphorus and inorganic carbon.
Photosynthesis and respiration (metabolism) in dense submersed aquatic plant stands cause daily fluctuations in the chemical content and pH of the water surrounding them. Aquatic plant stands change daily dissolved oxygen content in surrounding waters by as much as 12 mg/L . During daylight hours, while photosynthesis occurs, water can become supersaturated with oxygen. Respiration at night can deplete dissolved oxygen in dense beds with little water circulation. Metabolism of submersed aquatic plants can also influence concentrations of dissolved inorganic carbon, which in turn impacts pH. Aquatic plants remove inorganic carbon from the water by assimilation and the production of marl (carbonate deposits that encrust some aquatic plants). By removing inorganic carbon, aquatic plants stands can change pH by 2 to 3 pH units during a 24-hour period. Additionally, aquatic plants release several dissolved organic compounds into the water that contribute to the metabolism of bacteria and epiphytic (living on the plant) microorganisms that can also impact oxygen, inorganic carbon, and pH.
Aquatic plants and associated periphyton (algae that attaches to plants) can influence nutrient cycles. Phosphorus, for example, is removed from the sediment via plant roots and incorporated into plant biomass. Phosphorus is also removed from the water by plants and associated periphyton. When plant tissue dies, phosphorus is released and circulated, at least briefly, back into the water column. The extent and timing of this cycling can greatly influence phytoplankton growth. If nutrients are "tied up" in aquatic plant and periphyton biomass during the growing season, little is available for phytoplankton growth, and the water in the littoral zone may be clearer than in deeper open-water zones. In northern lakes, if the nutrients are released when plants die in the fall, water temperatures are usually cool enough that phytoplankton blooms, at least noxious ones, do not occur. If macrophytes die during the spring or summer, as often happens with herbicide treatments, however, nutrients are released at an opportune time for phytoplankton growth.
Aquatic plant death and decay also add organic matter to the lake sediment. Additions of organic matter to the sediment influence dissolved oxygen concentrations in the lake water to a greater or lesser degree depending on when the additions occur and how much matter is added. If large amounts of dead organic matter are deposited on the lake bottom under warm, still conditions (e.g., if large herbicide treatments are conducted in the summer), dissolved oxygen will decrease, and oxygen depletion may harm aquatic organisms living in the lake. In northern climates, oxygen depletion occurs under ice (when the air can not replenish oxygen supplies to the water) and can become a critical problem if the decaying vegetation is extremely abundant, often killing fish in the lake. These are referred to as "winter kills."
How important is the littoral zone to overall lake productivity and ecology? The importance of the littoral zone to whole-lake primary productivity (the rate at which algae and macrophytes fix or convert light, water, and carbon to plant tissue in plant cells) varies with the surface area and volume of the lake and the size of the littoral zone in that lake. Small lakes generally have more shoreline length per lake surface area, so the percentage of productivity contributed by the littoral zone in a smaller lake is higher when compared to open-water algal productivity. Thus, the importance of aquatic macrophytes and attached periphyton to the overall productivity of lakes generally decreases proportionately as lakes get larger and deeper. Some shallow lakes, however, are exceptions to the rule. In some instances, a shallow lake can also have a limited littoral zone with low submersed macrophyte abundance because of natural circumstances (low water clarity) or lake management activities (macrophyte control with herbicides, biocontrol, or mechanical harvesting). In these lakes, open-water algae would again dominate the total primary productivity of these systems.
Generally, the more productive the littoral zone, the more productive the whole lake is likely to be, if the definition of productive is carbon fixed (total photosynthesis). There are, however, few herbivores in North America (invertebrates or fish) that derive energy directly from aquatic macrophytes. Recently, stable carbon isotope analysis (an analysis that follows the flow of carbon through a food web from primary producers through top carnivores) in a shallow Florida lake showed that the carbon source for 12 species of fish and five species of invertebrates was primarily epiphytes (algae that grow attached to aquatic plants), and not eel-grass (Vallisneria americana), the rooted aquatic plant that covered 90% of the lake area. Thus, while eel-grass was fixing the majority of the carbon in the lake, the carbon fixed by the periphyton was the major source being transferred through the food web.

Credit: US Army Corps of Engineers

Credit: J. Butler, UF/IFAS Extension
The Biotic Component
Aquatic plants and attached periphyton in the littoral zone are food and habitat for a wide variety of organisms. Because this is a rather large and understudied topic, we will discuss it separately from the other effects that macrophytes have on their environment. The physical and chemical changes that macrophytes produce in the littoral zone impact the organisms that live there. We separate the relationships only for ease of discussion and will emphasize the relationships with epiphytes and macroinvertebrates, fish, and wildlife species.
Aquatic plants' influence on lake systems is tremendous. This circular cannot fully explain all aspects of their impact, but it will highlight some of the more important characteristics of aquatic plants and their place in the ecosystem.
Aquatic plants are colonized by a rich array of attached algae (periphyton) and microbes, particularly in hard-water lakes where carbonate deposits strengthen the matrix formed by the attached organisms. The total productivity of the attached organisms ranges from 4 to 93% of the host aquatic plant productivity. As mentioned above, open-water algae are sparse in the presence of abundant aquatic plants and attached periphyton. One reason for this is the competition for nutrients between periphyton and open-water algae; periphyton appear to be much more active than their host plants in dissolved-nutrient exchange.
High invertebrate densities, typically associated with aquatic plants, result in part from the abundance of periphyton (prime invertebrate food) available on macrophyte surfaces. Many invertebrates associated with aquatic plants eat the periphyton complex on the surface of the macrophytes rather than the macrophytes themselves. A few invertebrates, however, feed directly on aquatic macrophytes. A classic case is the denuding of some macrophyte communities in northern Wisconsin lakes by the exotic (for this region) rusty crayfish (Orconectes rusticus). Also, mining insects like the hydrilla tip mining midge (Cricotopus lebetis), bore through plant tissue, and some insects use plant tissue as habitat to lay eggs and nurture immature life stages (e.g., waterhyacinth weevils, Neochetina spp.). With these activities, insects destroy much more macrophyte tissue than they consume.
Invertebrates that live in sediments congregate beneath macrophytes because of the abundance of organic matter trapped or deposited by the aquatic plants. Some eat aquatic plant remains and others eat algae that cover the sediments. The total abundance of invertebrates (primarily chironomid/midge larvae) varied up to 196,000/ m2 on and under Eurasian watermilfoil beds in a cove of the Hudson River, New York. In the Eau Galle Reservoir in Wisconsin, scientists found ten times more bottom-dwelling organisms in a bed of coontails than they found in an adjacent barren area with the same substrate type. The inshore area under macrophyte beds in Halverson Lake, Wisconsin, contained 60% of the midge larvae and over 90% each of snails, fingernail clams, and caddisfly, dragonfly, damselfly, and mayfly larvae that existed in the lake. These examples again point toward the importance of aquatic plants to aquatic ecosystems.
The importance of aquatic invertebrates may not be obvious to many lake users. However, aquatic invertebrates are a major food source for forage fish and young life stages of many game fish. Many waterfowl and other birds also depend heavily on invertebrates as a high protein food source needed for reproduction and rapid early growth of their young. Without aquatic plants, the aquatic invertebrates, periphyton, and open-water phytoplankton that provide for the energy (i.e., food) needs of recreationally important fish and wildlife species would be absent from lakes.You can again see the importance of aquatic plants to lake systems.
Fish

Credit: Will Strong, UF/IFAS Extension
The interactions between fish and aquatic plants are highly variable because lakes themselves differ widely in morphology, trophic state, plant/fish species distribution and abundances, geographic area, and other characteristics. Generally, however, there are fish species that decrease in abundance (e.g., bluespotted sunfish, Enneacanthus gloriosus), increase in abundance (e.g., gizzard shad, Dorosoma cepedianum), and maintain the same abundance (largemouth bass, Micropterus salmoides) as aquatic macrophyte abundance decreases in lakes.
Each lake has a carrying capacity for the total amount of fish, which is primarily determined by the nutrient richness of the lake, or its trophic state. Within that carrying capacity, aquatic macrophytes can determine fish species and sizes in a lake. High aquatic plant abundance favors fish species that are adapted to aquatic plants (mostly small fish). Low aquatic plant abundance favors larger fish species that are adapted to open water. The number of species in a lake generally remains the same, and only the species composition changes as aquatic plants change in a lake. A good example of this is Lake Baldwin, Florida, which went from 95% covered with hydrilla to <1% after the introduction of grass carp, while maintaining the same native fish species richness (number of species).
A major factor determining the value of aquatic plants to fish is whether the fish is a prey species or a predator species. The presence of aquatic macrophytes increases the physical structural complexity of lake ecosystems. This structural complexity provides refuge for prey species and interferes with the feeding of some predator species. Exposure to predators strongly determines small fish feeding behavior and survival rates. If they are relatively safe from predators, they can forage more effectively. For large predators, the visual barrier of plant stems decreases their foraging efficiency; hence growth of large predators declines as habitats become more complex.
Sometimes small areas of littoral habitat, while not contributing significantly to the total production of the lake, are important for the reproduction or recruitment (i.e., spawning or nursery habitat) of some fish or other aquatic organisms. For example, although spawning on macrophytes is unusual for salmonids, at least a portion of the population of lake trout (Salvelinus namaycush) in Lake Tahoe spawns in deep water (40–60m deep) over beds of muskgrass (Chara spp.). No additional evidence of spawning was found over rocky formations that exist at various depths in the lake. Apparently, the muskgrass mounds, which represent a small portion of the primary productivity in Lake Tahoe, are favored as spawning habitat because they provide the basic requirements for successful egg incubation.
These are only a few of the important relationships that exist between aquatic plants and fish populations. Unfortunately, these relationships give little insight to how aquatic macrophytes affect "fishing." Some anglers enjoy fishing in and around aquatic plants and some do not, but most anglers agree that there can be too many aquatic plants for good fishing. Thus, the question boils down to how many plants are "the right amount" to provide habitat for fish populations and structure for anglers. Too few plants generally do not provide enough cover; too many may lead to stunted fish populations, poor predator growth, and poor access for fishing. The common answer is a moderate amount of aquatic plants. Several studies have suggested the optimum aquatic plant coverage in lakes for healthy fish populations ranges from 15–85%. Lakes with no aquatic plants and those with 100% volume infested with aquatic plants will both support fish populations. The problems with either no plants or too many plants are that some of these fish populations do not occur in the desired abundances or species compositions; and that it's difficult to fish in a weedy lake.
Wildlife
As with interactions between aquatic plants and fish, those between aquatic plants and wildlife are highly variable, again making the discussion of generalities difficult. Most fish are carnivores, but many wildlife species eat plants, and herbivory of macrophytes by wildlife causes much of the energy and nutrient transfer in the littoral zone. In Northern aquatic systems, Pelikan, Svoboda, and Kvet (1971) reported that 9%–14% of the net annual cattail production is consumed or used as lodge construction by muskrats. Smith and Kadlec (1985) reported that waterfowl and mammalian grazers reduced cattail production by 48% in the Great Salt Lake marsh. Muskrat grazing or "eat out" is important for maintaining diversity in the emergent zone. "Eat out" produces open areas in the cattail marsh that increase edge effect and allow submersed species and other emergent species to invade areas previously occupied by a single species of dense, emergent vegetation. In Florida, some species of turtles have been known to graze significantly on submersed aquatic plants.
Seeds, tubers, and foliage of submersed species are used as food by a variety of wildlife, especially waterfowl. Plant material is often high in carbohydrates, which provide energy for long migratory flights. Scientists estimated that waterfowl consumed 40% of the peak standing crop of sago pondweed in Delta Marsh, Manitoba. The scientific name of canvasback ducks (Aythya valisinera) shows their close association with wild celery or eel-grass (Vallisneria americana), which they eat in abundance during fall migration and on their wintering grounds in Chesapeake Bay. A major concern about the invasion of Eurasian watermilfoil is its ability to displace wild celery in large shallow lakes in Minnesota, Wisconsin, the upper Mississippi River, and Chesapeake Bay—traditional resting areas for canvasbacks, a species with generally declining numbers.
Invertebrates, produced in macrophyte beds, are also important to many wildlife populations. The invertebrates produce the protein that is vital to laying hens and chicks of many waterfowl and other waterbirds. Higher up the food chain, eagles, osprey, loons, mergansers, cormorants, mink, otter, raccoons, and herons, to name a few, feed on fish or shellfish that dined on invertebrates that lived in aquatic plant beds.
The emergent zone provides nesting sites and nesting materials important to species like red-winged and yellow headed blackbirds, marshwrens, grebes, bitterns, Canada geese, and muskrats. Sometimes the importance is not direct. Geese and other waterfowl sometimes nest on top of muskrat houses or muskrat food piles made of cattails.
Richness of bird species is positively correlated to lake surface area and trophic state of Florida lakes but not to aquatic plants (Hoyer and Canfield 1994). As aquatic plant abundance increases, however, birds that used open-water habitats are replaced by species that use macrophyte communities. Some bird species require specific types of aquatic vegetation, and removal of these types may exclude individual bird species from a lake system.

Credit: Mark Hoyer, UF/IFAS Extension
A topic seldom discussed is the ability of wildlife to import and recycle nutrients. Hoyer and Canfield (1994) estimated that phosphorus loads into 14 Florida lakes by birds ranged from 0.1% to 9.1% of the annual phosphorus budget, an amount they thought was insignificant. The nutrient inputs to a small lake by a few hundred resting Canada geese after feeding all morning in a nearby cornfield, however, may be a different matter. Nutrient budgets for each individual lake must be analyzed in order to determine the significance of wildlife inputs.
A wildlife relationship of special concern is that between aquatic plants and mosquitoes. Before the invention of pesticides, the removal of aquatic plants was the dominant method of mosquito control. Some aquatic plant management is still done for mosquito control. Certainly anything that causes stagnant water and offers protection from predators of mosquito larvae has the potential to support a mosquito nuisance. This includes temporary ponds, knotholes in trees, and old tires lying in the backyard. Where aquatic plants exacerbate these conditions, they may contribute to the mosquito problem. If water circulation and predators are present, mosquitoes are much less of a nuisance.

Credit: Eric Zamora, UF/IFAS
Section 2: Aquatic Plant Management Problems
A weed is any undesired, uncultivated plant that grows in profusion so as to crowd out a desired plant. ~Modified from Webster's New World Dictionary
Introduction
Aquatic macrophytes can be beneficial or problematic/invasive in aquatic systems depending on the defined uses of the aquatic systems (Table 1). Because lakes and reservoirs cannot be all things to all people, even the macrophyte abundance within a given lake can be beneficial or problematic depending on one's use of the lake or reservoir. Thus, defining the primary uses of a lake or reservoir is the first step when developing a lake management plan and determining if there is an aquatic weed problem.
Even when reasonable people join to help shape a management strategy for a water body, several elements inevitably come into conflict. Among the more obvious are differences in desired uses for the water from each of the various interest groups, and varying degrees of knowledge about water quality, fisheries management, and aquatic plant management options. Another important difference can be simply our own level of experiences with aquatic and wetland plant management problems.
It is probably safe to say that no two people see exactly the same things when they assess a water body. Long-term residents who have witnessed hydrilla or Eurasian watermilfoil mats come and go will probably react very differently than new arrivals to the neighborhood who have never before seen the dramatic changes that can occur as these weeds fill the water column of a lake. The loudest voices at the homeowner's association meeting may be from the members unable to remember how extensive the cattails were before the dredging project was undertaken. Others may simply have never recreated or lived around water before, and may be very unsure about exactly what constitutes a serious problem, and what is a normal occurrence. Imagine, for instance, what a visitor from Okeechobee, Florida thinks when confronted with the excellent, but very different looking bass habitat of Lake Casitas in southern California. "No grass, no bass" may be the southern cry but not when they are regularly catching 18-pound largemouth bass in 60–100 feet of water in Lake Casitas.
To further complicate the situation, things that look like problems may not be actual problems, and, conversely, seriously degraded conditions may appear benign and not attract any attention at all. We humans are extremely visually oriented, and easily impressed by changes that are striking to the eye but that in fact may be rather small in impact. If the number of cattails in a community lake doubles over a two-year period, for instance, that change will likely be noticed by many people, regardless of whether it indicates a minor, relatively benign re-invasion after a mechanical removal project or a more profound change to the lake. Striking visual changes are more likely to be seen and to cause consternation than the more subtle and probably far more important changes that may be taking place to the water chemistry of the lake. A community effort to learn about lakes generally and to collect and share accurate and trustworthy historical information about their own lake can do more than almost anything else to resolve concerns about "what is happening to the lake?" A water quality monitoring system like the citizen volunteer programs in New Hampshire, Vermont, Wisconsin, and Florida can yield valuable information to help guide lake management decisions.
One of the most important first steps toward creating a history for a lake and a plan for its future is to identify the many types of aquatic and wetland plant management problems that have arisen and that may arise in the future, both to inform ourselves about the many potential problems and their solutions, and to help recent arrivals to the lakefront gain a better understanding about how serious the lake's problems are—or are not. Note that all community concerns about plants in the lake must be taken seriously and handled with sensitivity: sometimes benign conditions that don't harm or that even improve the water quality or the overall health of the lake ecosystem may be considered unappealing or may prevent people's full enjoyment of the lake. These must be considered serious problems, regardless of whether they immediately impact the water quality. Finally, if a lake manager believes in a different management strategy than the user groups, it may ultimately be the politicians that determine the outcome. Recognizing that there is science, there is human experience, there are disparate interests, and that these are rarely isolated from each other, is an important part of learning about resolution of aquatic and wetland plant management problems.
Visible Problems
Blocked Lake Access
It is often easier to work with visible problems that appear in aquatic and wetland areas ( vegetation that blocks access to the lake, for example) than invisible problems (like dissolved oxygen depletion caused by an excess of aquatic vegetation). Many of the visible problems, however, are more social than biological in importance.
Access problems occur when emergent, floating-leaved, submersed, freely floating, or woody vegetation obstructs boat ramps or boat trails. Some of these problems are purely in the eyes of the user. For example, if someone dredges an area of shallow water with dense populations of cattails in order to construct a boat ramp, normal sedimentation processes will be compounded by boat and vehicle traffic and rapidly fill in the dredged areas—and the cattails will grow back as thickly as before. The returned cattails pose a real problem to the boating public, but they are not a problem for the water chemistry and related biology of the whole lake. An increase in vegetation does not indicate a problem in a lake unless it is linked to changes in water elevation, hydroperiod (seasonal water elevation), nutrient loads, or other variables—and it is only a problem then if the changes do not coincide with management objectives.
Organic Sedimentation
Sediment is the sand, clay, silt, and organic matter that forms the bottom of a water body. Organic sedimentation is the filling of reservoir and lake bottoms with decomposing terrestrial and aquatic plants (both phytoplankton and macrophytes). This problem may be more significant in warmer latitudes, where aquatic plant productivity is enhanced by warm weather. While little is known about organic sedimentation in most water bodies, some studies have measured a significant contribution made by aquatic plants (e.g., giant reed Phragmites spp., cattail, water hyacinth) to the accumulation of materials in a lake bottom. Thus, keeping aquatic plant populations low during the growing season can greatly extend the time before mechanical dredging might be necessary to keep water depth at the desired level.
Whether organic sedimentation is an ecological problem, a user problem, or both, depends mostly on the human uses for the body of water. Accumulation of aquatic vegetation in ponds, lakes, and bogs is perfectly natural: an integral part of the natural succession of shallow open water bodies to vegetation-covered wetlands, or even terrestrial vegetation. Active management is necessary to stop, reverse, or slow succession in lakes people want maintained as lakes. Compounding an already difficult management problem in an increasingly drought-ridden state, many of Florida's water bodies that are rapidly filling in are now dominated by invasive, non-indigenous plants. Biomass production by these species can be many times that of the native species that are reduced or eliminated from the sites because of competition. In maintained lakes, it's usually best to concentrate efforts on reducing non-native species because the consequences of native plant growth are usually both slower and less severe.
Non-native or exotic plant species are often deemed undesirable because of their growth potential and because they replace native species. There is, however, little hope of totally eradicating these exotic plants, Thus, aquatic plant managers need to work to minimize their potential harm to defined lake uses.
In a particularly interesting way, water control structures on many of our water bodies act to prevent natural processes that would otherwise remove decaying vegetation. Flood waters scour river channels and may act to remove accumulating sediment from larger rivers, whereas most large lakes and reservoirs act as sediment traps. Some individual water bodies, however, may be susceptible to scouring during exceptionally violent storms. The dramatic rainfall associated with hurricanes and other intense storms operates periodically to scour sediment from shallow lakes. Water control structures, however, are now designed to reduce this active process. In the same way forest managers must use controlled burns to clear potentially explosive overgrowth of the forest understory after decades of successful suppression of wildfire, lake managers are increasingly finding it necessary to use deliberate reduction of aquatic plant vegetation to replace the natural scouring we have successfully suppressed with water control structures.
Sediment accumulation frequently increases when aquatic plants become established. The movement and accumulation of sediments in aquatic systems are not mysterious: they follow standard laws of physics. Large amounts of water flowing quickly carry more sediment than slow-moving flows of smaller quantities of water. (Floods and fast-moving rivers are called "high-energy water," whereas slower flows are "low-energy water.") Large items settle more quickly than small items as water energy decreases. Sediments also tend to travel downhill, and depressions in the bottoms of water bodies tend to fill over time. Because sediment type greatly affects plant establishment and growth, invertebrate populations, and fish spawning and feeding, it is not surprising that small changes in sediment type and depth can affect a water body in a number of ways. Thus, potential impacts of aquatic plant management on sediment characteristics should be included in any assessment of aquatic plant management options.

Credit: Mark Hoyer, UF/IFAS Extension
Plant Piles
Unwanted piles of live or dead (decaying) vegetation along residential shorelines, on boat ramps, in swimming areas, and in commercial boating areas are common sources of complaint. Floating plants and plant parts are wind driven, and so commonly accumulate in downwind areas. Rooted plants sometimes break free during storms, and many of them slough off stems and leaves when water temperatures drop in the fall and winter. Some breakage of plant parts occurs with most species throughout the growing season. Large accumulations of plant parts can result from mechanical removal of aquatic or wetland plants if harvesters don't make an effort to collect plants after cutting them. Chemical control can act to shear off plants near the hydrosoil surface (the lake floor), leaving only the roots behind. The plants then float to the surface and drift to the shore, where they collect in unsightly rotting heaps. Even biological control with grass carp can produce large amounts of moving vegetation. Grass carp often grasp stems near the middle or bottom of the plant, feed on part of what is removed, and allow the uneaten parts to drift.
Accumulated vegetation can create odor problems and can provide breeding locations for mosquitoes and other disease-carrying organisms. Nutrients leaching from a decaying mound of vegetation may cause small local problems like algal blooms, but nutrient cycles in large water bodies are generally not altered significantly by concentration of plant biomass in small areas. For example, the Florida Fish and Wildlife Conservation Commission scraped approximately 1.2 million cubic yards of accumulated muck and plant material from Lake Tohopekaliga and piled it onto the lake bottom creating 29 islands with a total footprint of about 66 acres (Figure 15). Water quality monitoring data showed that these islands did not significantly change the whole lake water chemistry.
Under several environmental conditions, aquatic plants can form floating islands, sometimes called tussocks. Floating vegetation can also form a substrate for the germination and growth of other plant species, increasing the size of the islands. These floating islands can become large and complex, causing many problems of their own. Large floating islands have blocked boat ramps and boat trails and can shade out or uproot other plants beneath them. Floating islands pose problems for water control structures, especially during high water flow. Movement of water through the structure can be partially or totally blocked, and large islands are capable of removing some structures. This is especially important when considering flood control programs.
Blocked Water Management Structures
The number of ways that aquatic plants can cause problems with water management structures seems endless, and it may actually be endless given the continuing development of new types of water-management equipment. The simplest problems to imagine (though not necessarily the simplest to solve) are the problems that arise when the accumulation of aquatic plants block gates in either an open or closed position and prevent their movement, usually precisely when their proper functioning is most critical. Under normal everyday conditions, the failure of a water control gate to move as it was designed to move can result in minor amounts of water going where it is not wanted or not going where it is wanted. Failure of these gates during emergencies, however, can result in catastrophic losses of property from flooding (damage to crops and livestock, damage to buildings and equipment), or from drying (damage to crops and livestock, added expense for water treatment or alternative water supply), and the potential for loss of life. When maintenance crews are attempting to clear aquatic plant accumulations from the intakes of hydroelectric systems on canals, the expenses fill many categories, including overtime for crews, loss of hydroelectric generating capability, and added equipment requirements. Sometimes, even specially trained underwater dive teams are required.
As water measurement devices become more sophisticated, the harm done to the devices themselves but more importantly to the data they collect from excessive aquatic plant accumulations seems to be getting worse instead of better. Plant material that collects in some of the simpler measuring devices such as water wheels and measured gate openings creates an expense because it requires that someone physically remove the built-up vegetation to restore accurate readings. The problem has become much worse with the arrival of remote-sensing measuring devices and gate adjusters that are designed to eliminate the necessity of human monitoring. Often these more complex devices continue to collect and transmit readings even when plant accumulations are interfering with water delivery quantification—and no person is present to discover the error. Sophisticated hydroacoustic equipment used to measure water flow through measured weirs in canals is rendered worse than useless when aquatic plants accumulate. The measurements of these systems are spurious at best when aquatic plants are present.
Concern about aquatic plants and their impacts to water management structures often reaches a maximum during natural storm events. Floodwaters can float water hyacinth and other species into and out of areas where they do not normally accumulate. Impacts of the floodwaters are magnified by the additional load of aquatic vegetation, which tends to become attached to structures. In extreme circumstances, accumulation of aquatic plants can result in the tearing out of a control structure or removal of highway bridges. These situations rarely confront a lakefront property owner, but they can be important discussion points when explaining the benefits of controlling nuisance growth of aquatic plants, especially to utility and resource managers and elected officials.
Physical problems caused by aquatic vegetation can be colossal enough to shut down a power plant or modest enough to fill in a boat ramp; but even if an aquatic plant problem is relatively small, if it prevents or impairs the determined use of a water body, it merits attention and action by the manager of that water body.

Credit: Ken Langeland, UF/IFAS Extension
Biological Problems
Difficult as they are, physical problems of water bodies are usually relatively straightforward and solvable when compared with the issues related to plant and animal community ecology. Most aquatic organisms fall into three categories: 1) organisms that increase in abundance as aquatic vegetation increases, 2) organisms that decrease in abundance as aquatic vegetation increases, and 3) organisms that are unaffected by aquatic vegetation density. A good example of this comes from the aquatic bird populations that use lakes in the southeastern United States. Bird abundance and total species richness remain relatively stable as aquatic plant abundance increases in a water body, but birds that use open-water habitats (e.g., double-crested cormorant, Phalacrocorax auritus) are replaced by species that use aquatic macrophytes (e.g., ring-necked duck, Athya collaris). Some species, however, maintain a constant density as aquatic plant abundance increases in a water body (e.g., least bittern, Ixobrychus exilis). Thus, increasing aquatic vegetation in a southeastern lake is problematic to the person who enjoys watching double-crested cormorants feeding on shad, beneficial to duck hunters, and inconsequential to the photographer attempting to take a picture of a least bittern.
The above bird example can be repeated for individual species of plants, invertebrates, mollusks, reptiles, amphibians, fish, and mammals inhabiting aquatic systems. The question, "For what users or what species do we manage this lake?" becomes even more complicated when we consider exotic, threatened, and endangered species. Do we use all of our resources to try to eliminate exotic species, or do we realize they are here to stay and manage lakes for a defined use? Do we ignore all other flora and fauna and manage lakes to promote the reproduction and success of threatened and endangered species, and, if we do, will our efforts reduce biodiversity in lake ecosystems? It is not difficult to see why biological problems caused by aquatic plants are more difficult to define and attempt to solve than physical problems caused by aquatic plants.

Credit: Mark Hoyer, UF/IFAS Extension
Water Clarity
As shown in Figure 3, aquatic macrophytes in a lake have an inverse relationship with suspended solids in that lake and therefore a direct correlation with improved water clarity. As aquatic macrophyte abundance increases in a lake, suspended solids in the lake decrease. Suspended solids are the algal cells, dead organic matter, clay particles and other small particles that float in water and decrease water clarity in most reservoir and lake systems. There are several hypotheses used to explain the relationship between aquatic plants and suspended solids. One suggests that aquatic plants and attached algae compete for the nutrients that would otherwise be expressed as suspended algae (e.g., phytoplankton). Another suggests that aquatic plants stabilize sediments and reduce the re-suspension of nutrients that could be used by suspended algae. Stabilizing the sediments also reduces the re-suspension of dead organic matter and clay particles. Either or both of these or other mechanisms may be working independently or together to cause the relationship between aquatic plants and improved water clarity; in any case, the fact that the relationship exists has been documented many times.
The relationship between aquatic plants and water clarity needs to be explained to stakeholders and incorporated into aquatic plant management plans in order to prevent the management of a "too-many-plants" problem from causing a "murky-water" problem. Most people consider clear water a positive attribute in a lake, and when visibility decreases from 15 feet to 3 feet after eliminating some of the aquatic plants, people are very likely to decide that the old aquatic plant problem the lake manager solved was not as bad as the new reduced water clarity problem the lake manager created.
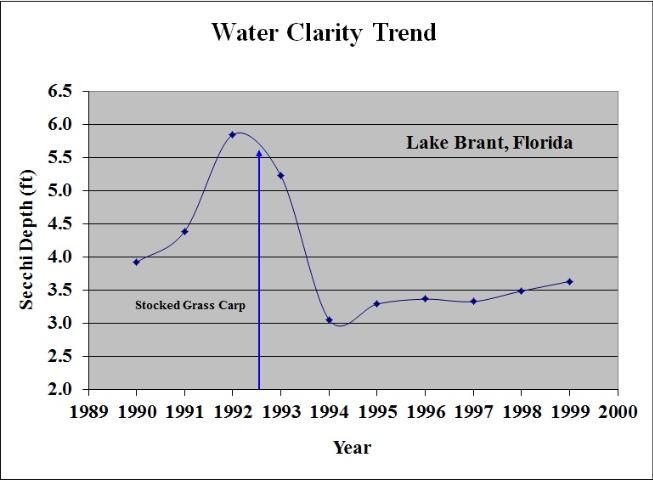
Controlling aquatic plants over 30% or less of a lake's surface area will not usually reduce its water clarity, but more ambitious efforts that remove plants over more than 50% of the lake surface will almost definitely reduce the clarity of the water noticeably throughout the lake. Significant reductions in water clarity usually occur only when whole-lake aquatic plant control programs are initiated. The use of grass carp is a good example of a whole-lake control technique. In almost all cases, assuming they are stocked in the lake in sufficient numbers, grass carp will control all the aquatic plants in that lake, and, in lakes with 30–50% aquatic plant coverage, a significant decrease in water clarity can be reliably predicted (Figure 18). Other aquatic plant management techniques (e.g., herbicides) will also reduce water clarity if they eliminate most of a lake's aquatic plants.
Fishing
The impact aquatic plants have on fish populations is visible and can be measured given sufficient money, equipment, and time, and aquatic plants' impact on catch and release or catch and harvest of sportfish is also visible (no fish, no boats) but not so easy to measure. Anglers who are used to fishing the edge of water hyacinth and hydrilla mats for largemouth bass are usually disappointed when those habitats are controlled and their catches decrease. Other anglers, however, may be pleased by or indifferent to aquatic plant control efforts. Largemouth bass anglers, for instance, who fish by trolling crank-baits in open water, will not see a reduction in catch and may find it easier to navigate the lake. The impact of a lake's aquatic plants on its recreational and sport fishing possibilities can be an extremely complicated question. Fish population biology , the availability of specific fish species, and the popularity of specific angling techniques in the area must all figure into the equation. A lake may support high numbers of largemouth bass, and many of them may be trophy-sized fish, but if the lake doesn't permit physical access to the areas where bass congregate, or if some aspects of the lake prevent anglers from using the fishing methods best suited to catch largemouth bass, it won't be a good lake for sportfishing because catch will be low.
Largemouth bass are typically found in or around topographical features on the lake bottom such as changes in slope, dead trees, etc. where they find most of their prey. When rooted aquatic plant coverage in a lake is high, largemouth bass leave their refuges to forage in openings in the weed mats. Fishing the openings in dense weed mats is a standard successful practice used to catch bass in many reservoirs and lakes. If vegetation is reduced, however, bass will return to topographical refuges. In that situation, the more successful anglers abandon the weed mats and follow the fish. Successful anglers adapt as conditions change, and take of sportfish frequently has more to do with angler patterns than with the dominance of the aquatic vegetation present in a lake or the number or type of aquatic plants. The presence or absence of aquatic vegetation in a lake, at least in the case of largemouth bass, is only a problem if the angling population views it as such.
There are several cases, though, where aquatic vegetation can be a problem to all fishing regardless of the methods anglers use. Most of these are physical blockages of access for people with boats or blockages of bank fishing for people without boats. Aquatic macrophytes can also cause fish kills by contributing to oxygen depletions, and it is difficult to catch fish when there are few fish in the lake.
Credit: UF/IFAS Center for Aquatic and Invasive Plants
Invisible Problems
Most physical problems caused by aquatic plants are visible and easy to define and solve. Most biological problems caused by aquatic plants are also visible, but comparably difficult to define and solve. Invisible problems, like insect-borne diseases that could be linked to aquatic plants may be the most difficult aquatic plant problems to define and resolve. Aquatic plants can also change water chemistry slightly, yielding invisible behavioral changes in the biological components of an aquatic system. For example, abundant aquatic vegetation can decrease dissolved oxygen in the water, changing fish feeding patterns with impacts that may cascade through an entire aquatic system. These are difficult problems to understand, let alone incorporate into a management plan. Thus, it is important for all parties helping to develop a management plan to have at least a general understanding of changes in the water that we can't "see," why and how they are measured, and what the measurements tell us.
Insects, Diseases, and Other Problems
Each year, a number of cases of equine encephalitis are reported to disease centers in the United States. This disease, along with several other equally dangerous diseases, is carried by mosquitoes. Mosquitoes and other insects find suitable breeding sites in slow-moving waters found in many aquatic systems. Mosquito-eating fish and other predators in a lake keep populations of mosquitoes and other biting insects lower by eating their eggs, larvae, and emerging adults, before become biting, disease-vectoring adults.
Aquatic plants can provide excellent hiding areas for mosquito and other pest-insect larvae in slow-moving water. Roots of water hyacinth often shelter numerous organisms, and thick mats of submersed vegetation can screen prey from hungry fish and invertebrate predators. Reduction of thick aquatic plant growth may not reduce the number of eggs laid in a particular area, but it may allow small fish and invertebrates the opportunity to feed on mosquito eggs, larvae, and emerging adults.
Insect problems related to aquatic plants are not always as "invisible" as they might seem at first. A few simple observations can often identify the types and general amounts of larval insects in a water body. In many parts of the country, mosquito control districts assess mosquito levels, and staff of these organizations may be available to assess the water body in question. Some districts also measure levels of insect-vectored diseases within populations of mosquitoes and other pest insects.
"Swimmer's itch," an occasional problem for lake managers, is probably best described as a collection of skin irritations that develop after contact with lake water. The organisms that cause swimmer's itch are highly varied, but some, at least, can be controlled with a sound lake-management plan that reduces the aquatic plant harborages of their host organisms. One of these, a parasitic flatworm, or trematode, must parasitize first an invertebrate, like a snail, a clam, or a worm, and then a vertebrate animal in order to complete its life cycle. At one stage in the cycle, the free-swimming larval trematode, called a cercaria, actively seeks to penetrate a host, which is usually a bird or fish, in order to form the next life stage (the metacercaria, which is a bit like a pupa). It's at this stage that the trematodes become a problem for people because the free-swimming cercaria don't distinguish between organisms they encounter. They burrow into whatever they run up against, and they are able to penetrate human skin just enough to cause a reaction. Trematodes cannot actually parasitize humans, but they do cause problems: sometimes a physical reaction to the attempted invasion, and in some susceptible people, an actual allergic reaction. Populations of the snails that support trematodes during the early life stage are often high in reservoirs and lakes with large aquatic plant populations. Control of aquatic plants is often used as a first step in reducing the human health hazard of this collection of organisms.
Dissolved Oxygen
Because we breathe air and because our atmosphere mixes thoroughly and easily, humans don't often stop to think about the distribution of oxygen and its levels of availability, that is, until we climb to 10,000 feet of elevation or higher or clamber down a mineshaft to try to rescue a miner stricken with "choke damp." Most of the time, even in fairly confined spaces (cars, homes, closed offices), enough air is exchanged with the "outside" to keep oxygen deprivation from being a problem.
It is a different story in water. Oxygen moves slowly through liquids, and even more slowly through solids such as ice. If the oxygen level in a small, deep reservoir could be indicated by various shades of blue (dark blue = very little oxygen, light blue = oxygen rich), the bottom layer of the reservoir might be almost black, indicating no oxygen at all. We could see fish and invertebrates avoiding the oxygen-depleted water; possibly moving into it briefly, but then moving to more oxygenated water very quickly. If we could somehow get very close to the black water, we might also see small organisms, even some fish, dying as they lose muscle control before they can move into better water.
How do aquatic plants affect oxygen concentrations in a water body, and can they cause a problem? A difficult concept to grasp for many people, is that plants need oxygen just as animals do, and plants can also die under very low oxygen conditions. We all learned in grade school that plants photosynthesize (produce their own food from sunlight, carbon dioxide, and an impressively complex set of associated chemical products, enzymes, and reactive surfaces), a process that yields oxygen as a by-product. If that's true, why would plants need oxygen? The answer is simple: they need oxygen for exactly the same reasons that animals need oxygen, to allow the complete breakdown of energy storage products (sugars and starches) to release chemical products for growth, and energy for chemical reactions (respiration).
Plants use carbon dioxide and sunlight to photosynthesize energy storage products (sugars and starches), but to use those products efficiently, they, like animals, must have access to oxygen. In a 24-hour period under situations of low light on cloudy days, the amount of oxygen plants use in respiration exceeds the amount they produce in photosynthesis. If the situation persists, oxygen depletions can occur, causing sometimes drastic harm to all organisms in the area. Managing aquatic plants at a moderate abundance can reduce the probability that they will cause oxygen depletions during long spates of cloudy weather.
As always, however, incomplete management will likely make a problem worse or create an entirely new problem. Aquatic plant controls that leave dead plants in the lake can create a greater oxygen-depletion problem than the plants posed in the first place. Dead aquatic plants no longer supply oxygen through photosynthesis, and bacteria use oxygen as they break down the aquatic plants, worsening the oxygen depletion problem. An effective aquatic plant management plan arranges for the removal of dead plants from the aquatic system.
Section 3: Aquatic Plant Management Techniques
Anyone looking for a solution to a lake plant problem should first determine what state agency or agencies are responsible for aquatic plant management in the area and then contact the agencies to determine what assistance is available and what courses of action are legal for private citizens. Problems that inhibit access and use of a public lake are in most cases the responsibility of a public agency. Decisions concerning perceived whole-lake problems on private lakes should be addressed through the consensus of a home-owner's association after obtaining recommendations from public agencies.
Whole-lake aquatic plant problems are generally managed by public agencies. Sometimes, these aquatic plant problems are handled by commercial management firms that have the necessary equipment and expertise to solve the problem. Management of aquatic vegetation in small areas along private beaches or around boat docks may be accomplished by the individual property owner, although even in these situations it usually is best to obtain the services of an experienced aquatic plant manager. It is essential for an individual who decides to conduct his or her own aquatic plant management to determine what can be legally done. If herbicides are used, the plants must be properly identified, and it is essential to use only herbicides that are registered for use in aquatic sites and to become fully trained in their use. This information should be available from a county Cooperative Extension Service Office, state natural resources agency, or state department of agriculture.
The diversity of lake types dictates that commercial and public aquatic plant managers, as well as individual waterfront property owners, carefully choose the most appropriate method or combination of methods to manage aquatic plants for each individual situation. The effectiveness and benefits of methods used for controlling the pest plant must be weighed against potential impacts on non-target plants and animals and impacts on water uses such as swimming, fishing, irrigation, livestock watering, and domestic consumption. The following methods for managing aquatic weeds are most often used:
- Physical removal
- Habitat alteration
- Biological controls
- Herbicides

Credit: UF/IFAS Center for Aquatic and Invasive Plants
Physical Removal (Harvesting)
Hand Removal
Simply weeding by hand may be all that is necessary to remove small amounts of vegetation that interfere with beach areas or boat docks. Of course, hand removal is labor intensive and must be repeated routinely. The practicality of this simple and effective method will depend on availability of labor, the regrowth or reintroduction potential of the vegetation, and the level of control desired.
Regrowth of vegetation depends on which species of plants grow in the lake, nutrient levels in the lake, and the seasonal growth trends of plants. Cattails and many grasses, which can reproduce from small root fragments, require frequent removal because it is impossible to remove these plants without leaving root fragments in the sediment, which sprout new growth. Most aquatic plants tend to grow rapidly in the spring, slowly in the fall, and very slowly or not at all in the winter. This growth pattern becomes more pronounced as one moves from southern to northern climates.
Introduction or reintroduction of new plants can result from natural seed dispersal; plant fragments generated naturally, by boat traffic or by the removal efforts; wind or current dispersal of floating plants; or spread by waterfowl and various human activities.
Frequency of hand removal will depend on the combination of factors for each individual situation. For example, weekly removal of water hyacinth plants may be necessary from a boat dock area on a productive Florida lake, but a single spring removal of grasses may be all that's needed to maintain the beachfront of an unproductive Wisconsin lake.

Credit: Ken Langeland, UF/IFAS Extension
Hand removal for control of aquatic vegetation may be used in combination with other methods such as herbicides or benthic barriers to minimize regrowth. However, hand removal has the distinct advantage that it can be very selective for removing undesired vegetation while maintaining desired plants.
Mechanical Removal
Specialized machines are available in many sizes and with several different accessories for removing aquatic vegetation in a variety of situations. Small machines are practical for limited areas, and large machines in combination with transports and shore conveyors are suitable for large, whole-lake operations. These machines are commonly called mechanical harvesters or weed harvesters, and the process is called mechanical harvesting or removal.
In certain circumstances, such as cutting boat trails through dense stands of vegetation, mechanical removal has several advantages over other methods. Immediate control can be achieved in small areas. Treated areas can be used immediately, whereas in areas treated with herbicides, water-use restrictions may apply. Mechanical removal minimizes the objectionable dead and dying vegetation that may be associated with other methods.
Several disadvantages limit the use of mechanical removal for aquatic weed control in many regions, however. It usually costs more and is slower and less efficient than other methods, and there are high maintenance and repair costs for the machinery. Shallow water and obstructions render some water bodies unsuitable for mechanical removal operations. Plant fragments easily drift to infest new areas. Mechanical removal disturbs sediments and temporarily increases turbidity. A suitable area for disposal of harvested plants must be available. Finally, this method is imprecise and routs wildlife (e.g., small fish, snakes, newts, turtles) and desirable vegetation along with the weeds.
Dredging
In extreme cases of overgrown aquatic vegetation, conventional or specially adapted dredging machines may be used to remove vegetation and associated sediments. Dredging is expensive, especially if a nearby disposal site is not available. The secondary environmental effects of dredging can be quite drastic, and therefore permits from regulatory agencies must be acquired before a dredging operation can begin. Following dredging, other methods should be used to maintain vegetation growth and prevent recurrence of the extreme situation. Dredging is the most comprehensive management solution—it is not possible to "spot treat" with this method. Unless dredging covers the entire rim of the lake to a point just beyond the photic zone (the area of a lake where photosynthesis can occur, defined by the depth to which at least 1 percent of the surface light intensity penetrates), its beneficial effects will usually be short lived.
Habitat Alteration

Credit: Julie Terrell, UF/IFAS Extension
Water-Level Manipulation
Water-level manipulation refers to the deliberate raising or lowering of water levels to control aquatic vegetation. Raising the water level drowns plants, and lowering the water level exposes them to freezing, drying, or heat. This method is limited to lakes and reservoirs with adequate water control structures.
Drawdown, the lowering of lake water level, is more commonly used than raising water levels. Drawdown has been used in lake management for many years to oxidize and consolidate flocculent sediments, to alter fish populations, and to control aquatic weeds. In addition to the need for an adequate water control structure, use of drawdown for aquatic plant management may also be restricted by considerations such as water-use patterns and water rights (e.g., disruption of recreational or agricultural use) or a predictable source of water for refilling.
Drawdown is usually conducted during winter months so that plants are exposed to cooler, dryer weather, which tends to accelerate drying. Summer drawdown can also be effective but usually results in greater impact to agricultural and recreational water use, stresses fish populations, and has a greater potential to enhance the spread of emergent plants such as cattails, rushes, and willows.
Drawdown alters the composition of aquatic vegetation (different plants will grow in the lake) but the changes it produces are not always desirable. The responses of various aquatic plant species to drawdown vary widely (Table 2) and sometimes unpredictably. Brazilian elodea (Egeria densa) is sensitive to drawdown and is often controlled for up to three years by this method. In contrast, drawdown only partially controls hydrilla, a near relative of Brazilian elodea, and only when it is growing in sandy lake bottoms; it has little effect on hydrilla when it is growing in organic sediments. Hydrilla tubers that are produced deep within the sediment are protected from desiccation and can survive several consecutive drawdowns. In general, submersed aquatic plants have variable responses to drawdown, while emergent plants tolerate or are stimulated by drawdown.
The advantages of drawdown as a method of aquatic plant management include low cost (unless recreational or power generation is lost) and the secondary benefits of sediment oxidation and consolidation and fisheries enhancement. Potential undesirable effects of drawdown include reductions of desirable species, increases of undesirable tolerant species like hydrilla, expansion of undesirable species to deeper areas, the creation of floating islands, and the loss of storage water and recreational benefits if insufficient water is available to refill the basin.
Reduction of Light Penetration
All plants require a certain amounts of light to grow. Submersed aquatic plants can sometimes be controlled or suppressed by reducing light penetration into the water. Light penetration can be reduced by the use of special dyes, special fabric bottom covers, fertilization, and/or raising water level.
Only those dyes that are approved for use in water should be considered. These specially produced dyes block light that plants need for photosynthesis and are not toxic to aquatic organisms, humans or animals that might drink the treated water. Dyes are only effective in ponds that have little or no flow through them, and they are generally effective only in water greater than 3 feet in depth.
Various materials, including black plastic and specially manufactured bottom covers, have been used to prevent rooted aquatic plants from growing. Gases that are produced on pond bottoms accumulate under plastic and other nonpermeable bottom covers and cause them to float to the surface. However, specially made bottom covers can be effective for preventing submersed aquatic plant growth. In addition to preventing light from reaching the pond bottom, these materials also physically prevent rooted aquatic plants from becoming established. These special materials are expensive and must be maintained to prevent sediment accumulation on top of the cover. Therefore, their use is generally restricted to ornamental ponds, swimming areas or areas around boat docks (in these areas care must be taken to prevent the bottom cover from becoming tangled in boat propellers).
Fertilization increases the growth of algae that in turn decreases light penetration and limits the depth at which submersed aquatic plants can grow. Similarly, raising the water level increases the distance light has to travel to the lake bottom, and if the water clarity is the same then the depth for aquatic plant growth also decreases.
Nutrient Limitation
Most plants need nitrogen, phosphorus, and carbon to grow. Theoretically, reducing at least one of these nutrients could keep aquatic plants from growing to an objectionable level In actual lakes, however, unless is they are extremely oligotrophic (nutrient poor), the sediment will contain sufficient levels of nitrogen, phosphorus, and carbon to sustain abundant rooted aquatic plants.
In some areas, nutrients are naturally in short enough supply that aquatic plants do not grow to problem levels. Where human inputs have accelerated plant growth, nutrients can be limited by identifying and abating the nutrient source(s). If the lake has received external phosphorus inputs for a long period of time, it may also be necessary to reduce internal nutrient availability by precipitating phosphorus to the bottom with agents such as alum. While nutrient limitation is theoretically possible, there is as yet no evidence that it actually works. No good examples of nutrient limitation resulting in effective control of nuisance populations of aquatic plants exist in the literature.
Consider, too, that nutrient control efforts may actually aggravate an existing aquatic plant problem. There are well-documented cases where nutrient limitation controlled planktonic algae populations and increased light penetration to the sediment, all of which allowed aquatic plants to expand their coverage in the lakes and reservoirs.
Biological Control
Biological control is the purposeful introduction of organisms, such as insects and pathogens, to keep the growth of problem plants in check. Biocontrol agents have to be released into the problem plant's range to help suppress its growth. Small numbers of biocontrol agents are released so that they can increase to a point where they control the problem plant and are in balance with the target plant, so a self-perpetuating population is established. In some cases, like that of the milfoil weevil, a native insect shows a preference for the exotic nuisance plant over its previous plant habitat and helps control the exotic species.
The most attractive aspect of biological control is that it can be permanent and self-perpetuating. Once established, additional releases are usually unnecessary, so additional expenses are avoided. However, exceptions occur when it becomes necessary to move field-collected bioagents to new locations. While the initial expense is high, over the long run, biocontrol agents are among the least expensive control options. Benefit-to-cost ratios of this approach have been estimated at 50–100:1 or even higher.
A foreign insect species must be extensively tested and proven to be host-specific (that is, it must be shown that the organism cannot reproduce in the absence of the exotic host) before it can be released in the United States. These tests are designed to demonstrate that the bioagent will not feed appreciably or reproduce on any plant other than the target weed. This ensures that it will not harm crop plants or other desirable species.
The first aquatic weed target for biocontrol in Florida was alligatorweed (Alternanthera philoxeroides). Three host-specific South American insects were found and eventually released. These include the alligatorweed flea beetle (Agasicles hygrophila), which was released in 1964; the alligatorweed thrips (Amynothrips andersoni), which was released in 1967; and the alligatorweed stem borer (Vogtia malloi), a moth, which was released in 1971. These insects are very effective and usually suppress the growth of alligatorweed below problem levels. However, their effectiveness is diminished toward the northern limits of the plant's range in North Carolina. These insects are naturalized throughout the southeastern United States, but populations sometimes are diminished following harsh winters. When this happens, control can be enhanced on a localized level by importation of insects from more southerly regions.
Three species of insects have been released for control of water hyacinth. The first was the mottled water hyacinth weevil (Neochetina eichhorniae), which was released in Florida in 1972. The second was the chevroned water hyacinth weevil (Neochetina bruchi), which is quite similar to the first. It was released in Florida in 1974. The third insect was a moth, the water hyacinth borer (Sameodes albiguttalis), which was released in 1977. These three insects are naturalized throughout the Southeast. A good indication of the presence of water hyacinth weevils is the occurrence of distinctive adult feeding scars on the leaves. Mature larvae can often be found in the petiole bases or in the stem. The weevils (especially the chevroned) have been the most effective of the water hyacinth insects. It has been difficult to quantify the impact of these insects on water hyacinth populations, but suppression has not been sufficient to diminish the need for aggressive maintenance management of water hyacinths with herbicides.
Several insect biological controls are in various stages of research, quarantine, and early release for control of water lettuce (Pistia stratiotes), hydrilla (Hydrilla verticillata), and Eurasian watermilfoil (Myriophyllum spicatum). The interested reader is urged to contact an information source such as the University of Florida/ Institute of Food and Agricultural Sciences, Aquatic Plant Information Retrieval for current information on biological control progress (APIRS UF/IFAS Center for Aquatic and Invasive Plants, 7922 NW 71st Street, Gainesville, FL 32653-3071; https://plants.ifas.ufl.edu/).
Pathogens
Introducing plant pathogens might seem like a good method for control of aquatic weeds, but this method is somewhat limited by restrictions on the importation of plant pathogens from abroad. Regulations tend to prohibit this approach and limit the scope to native pathogens. Pathogens also tend to be environmentally sensitive, and pathogen populations often do not remain high enough for sustained suppression of weed populations. Pathogens show some potential for use as an augmentation along with other control methods. Suspensions of fungal spores can be formulated and applied to weed populations. One fungal pathogen (Cercospora rodmanni), has been formulated as a mycoherbicide for water hyacinth. However, it has not been very effective. Research is also currently being conducted to develop methods for biological control of hydrilla and Eurasian watermilfoil with pathogens. In the natural world, insects, especially stem borers and piercing- sucking types, often provide points of entry for native plant pathogens. While neither the insect nor the pathogen has a substantial impact on the nuisance plant population alone, in combination they provide some control of nuisance situations.
Credit: UF/IFAS Center for Aquatic and Invasive Plants
Snails, Manatees, etc.
Two snails (Marisa cornuarietis and Pomacea australis) have been studied as potential biocontrol agents for aquatic weeds. Large numbers will control several species of submersed aquatic plants under confined conditions. However, snails are not currently under consideration as biocontrol agents for aquatic weeds because of environmental risks associated with the purposeful propagation of prolific, generalized herbivores. They are also intermediate hosts for certain fish and human parasites, and they are not effective under natural, unconfined conditions.
Populations of the exotic channeled applesnail (Pomacea canaliculata), a larger relative of the native Florida apple snail (Pomacea paludosa), were thought to be exploding in many locations around the state but in fact applesnails are difficult to distinguish, and the snail in question was not P. canaliculata.(Fasulo 2011) At high densities these snails have the potential to consume excessive amounts of aquatic plants, but they must not be introduced as a control measure for aquatic weeds because in this case the cure would be substantially worse than the disease. Channeled applesnails in China were shown to carry rat lung worm, a parasite that can cause paralysis and blindness and sometimes death in people (and rats) (Capinera and Walden 2013). Exotic applesnails may displace Florida's native applesnails from their habitats by competing for food. Furthermore, channeled applesnails eat not only problem plants but also rice and other crops (Capinera and White 2011).
Manatees, or sea cows (Trichechus manatus), have been experimentally used, mainly in canals, for aquatic weed control in Florida. Manatees effectively removed submersed and floating plant species. During winter, however, heaters were required to keep the manatees warm. In a study of King's Bay (Crystal River, Florida) conducted by the US Fish and Wildlife Service, biologists found that 10 times as many manatees as normally wintered there could not consume the existing hydrilla biomass, much less keep up with the growth of plants.
Other biological controls for aquatic weeds that have been suggested and/or tested include ducks, geese, crayfish, nematodes, viruses, and water buffalo. Any of these may be useful under highly specialized conditions, but none have proven practical. Some of these agents may also cause more harm to aquatic systems than any aquatic plant nuisance. For example, the rusty crayfish (Orconectes rusticus) has denuded some northern lakes of plants vital for fish habitat, and it preys on fish eggs.
Triploid Grass Carp
Grass carp (Ctenopharyngodon idella) are the most commonly used and effective biological control for aquatic plants currently available. The success of grass carp is also the primary reason this biocontrol agent is so controversial. If stocked at a high enough densities, grass carp can remove virtually all aquatic vegetation in a lake for a decade or longer. Because of the fear that grass carp could escape and reproduce in open waters, most states that allow grass carp for aquatic plant control require that they be sterile triploid fish. (Triploid species have three sets of chromosomes instead of the normal two, which makes them infertile.)
Triploid grass carp are produced in hatcheries and, because of their sterility, are the only non-indigenous fish that can be legally used for aquatic weed control in most states. A permit is usually required for possession and use of triploid grass carp. Because they cannot reproduce, the number of fish will not increase beyond the initial stocking. However, they cannot be effectively removed from large bodies of water, and they are often hard to contain.
Triploid grass carp prefer to consume submersed plants, so they are effective controls of this type of vegetation. Grass carp also browse tips of young, tender emergent plants. This browsing behavior often limits emergent species, some of which may be non-target species. Although young grass carp feed on filamentous algae such as Cladophora and Spirogyra, they will not provide effective for control of most filamentous algal species unless all other aquatic plants are gone and unless the grass carp are stocked at high rates (>50 per acre). Grass carp do not control phytoplankton.
The ability of grass carp to consume aquatic plants depends on the size of both plants and fish. Factors such as age, gender, and population density of the fish can determine the consumption rate of the stocked fish. The species, abundance, and location of the aquatic vegetation also influence the feeding behavior of the grass carp.
Because predators like birds, snakes, other fish, and some mammals prefer smaller, more manageable fish first, it is best to stock grass carp that are 1 pound (10–12 inches) or larger to maximize their survival. Some fish will die even if only larger fish are stocked; therefore, it is not possible to know exactly how many fish remain in a pond or lake after it is stocked.
Stocking rates of 20–25 grass carp per acre of lake effectively control all aquatic plants in southern latitudes, but rates as high as 150 grass carp per acre are required before similar control is achieved in northern lakes. At any latitude, if enough grass carp are stocked that the consumption rate of the grass carp exceeds the growth rate of the aquatic plants, grass carp are an effective method of controlling aquatic vegetation (except for a few non-susceptible species, such as spatterdock, Nuphar luteum). Because of their nonselective feeding behavior and lack of predictability, grass carp should only be used in lakes where complete control of aquatic plants is an acceptable part of a management plan.
Many management agencies are currently attempting to use low stocking densities of grass carp (2–5 per acre) in combination with herbicides to control nuisance aquatic plants, while maintaining certain levels of aquatic vegetation. Because of the dynamic nature of aquatic systems and the inability to determine mortality rates of grass carp after stocking, this technique is unpredictable and should only be used with the understanding that of the end result may be the total eradication of all aquatic plants in the water body.
As yet no evidence demonstrates that it is possible to keep some submersed aquatic vegetation in a lake stocked with grass carp, even at low densities. This explains the common warning in the grass carp literature that "unless complete elimination of submersed aquatic vegetation can be tolerated, grass carp stocking is not recommended." A key element of a grass-carp plant management plan, therefore, is a cost-effective strategy to remove the fish from the system when they achieve plant control in the goal range and before they exceed the target. Unfortunately, however, over the several years since triploid grass carp were made available for plant management, lake managers have experimented with several methods for removing grass carp from lake systems, and none have proved successful. The methods tried included herding, angling, attracting, using lift nets, and killing the fish with toxic baits. Unfortunately, all techniques used in the removal studies were time consuming, labor intensive, and sometimes quite expensive. Worst of all, in each case, the methods failed to remove a major portion of the grass carp population. This is especially important in light of evidence suggesting that it may take only 0.5 grass carp per acre to eliminate regrowth after the fish have consumed all the submersed vegetation in the lake.
Ongoing research designed to develop an implantable device that would limit the lifespan of stocked grass carp may make grass carp a more predictable and thus more useful tool for managing aquatic plants. The ability to manage the life-span of grass carp would give them much greater short-term utility and reduce the potential for overstocking and wiping out all the vegetation in a lake for decades after the introduction of the voracious fish. The device would also allow lake managers to control their dispersal in the event of an escape by quickly dispatching runaway grass carp before they could disperse to other waters.

Credit: Jerome Shireman, UF/IFAS Extension
Tilapia
Tilapia are tropical species that can suppress growth of softer aquatic vegetation such as filamentous algae and bladderwort (Utricularia spp.) when stocked at high density (300 per acre). Two species of tilapia have been considered for aquatic weed control. The blue tilapia (Oreochromis aurea) feeds entirely on algae (planktonic and filamentous) but does not readily consume larger, coarser vegetation. The redbelly tilapia (T. zilli) feeds on larger submersed vegetation rather than algae. However, both species reproduce rapidly and consume not only vegetation but many small animals that are important food sources for desirable fish populations. Therefore, use of tilapia can have unwanted environmental consequences.
Tilapia will not overwinter in water below 43°F to 65°F. This is a benefit from an environmental standpoint, but annual restocking is necessary in temperate climates unless a warm water supply (such as a thermal spring or power plant cooling effluent) is available as a refuge during winter. In tropical climates, where they do overwinter, tilapia are prolific and can be detrimental to sportfish populations.
Before stocking any type of biological control of aquatic weeds, you must check with the appropriate state agencies to determine state regulations!
Herbicides
What are herbicides?
Most people asked to define "herbicide" would come up with "weed killer." Weed scientists define herbicides more precisely as chemicals used for killing plants or severely interrupting their normal growth processes. For the aquatic plant manager or waterfront homeowner, herbicides are useful tools that, used properly, can safely, efficiently, and inexpensively manage aquatic vegetation. A herbicide formulation consists of an organic (carbon-containing) or inorganic active ingredient, an inert carrier, and perhaps adjuvants/surfactants (wetting or spreading agents).
Herbicides must be registered by the Environmental Protection Agency (EPA) for use in the United States. There are about 200 herbicides (active ingredients) currently registered in the United States. Currently, only 13 are labeled for use in aquatic sites: bispyribac, carfentrazone, copper, 2,4-D, diquat, endothall, flumioxazin, fluridone, glyphosate, imazamox, imazapyr, penoxsulam, and triclopyr. Of the 13, only fluridone is exclusive to aquatic use. All of the other compounds are used in terrestrial environments. Some of them can be used on food (glyphosate on Roundup Ready crops, carfentrazone and triclopyr on rice) and some in forestry and on rights of way (glyphosate, triclopyr, 2,4-D, and imazapyr). With all of these terrestrial and aquatic uses, it remains very important to use only those compounds that are labeled for aquatic use. Use of a herbicide that does not specify aquatic sites on the label is a violation of law.
The reason there are few aquatic herbicides compared to crop-production herbicides is that the aquatic environment has several unique characteristics that render it more vulnerable and also more difficult to treat. These factors set limits on the number of compounds that will both effectively control aquatic plants and also meet the rigid environmental and toxicology criteria necessary for registration. Aquatic herbicides must be taken up by plants quickly and in sufficient amounts to be toxic to target plants but they must also have sufficiently low toxicity to people and organisms in the aquatic environment to make them safe for use. The market for aquatic herbicides is also small compared to the giant market for herbicides used in agriculture. With this said, there are currently several new herbicide compounds being evaluated in the aquatic market. These new compounds tend to be plant enzyme inhibitors that exhibit very low toxicity to fish and wildlife.
The Herbicide Label

Before a herbicide is labeled by the United States Environmental Protection Agency (USEPA), extensive research that requires many years to complete must be conducted. In addition, aquatic herbicides that were registered prior to guidelines that were established by 1978 amendments to the Federal Insecticide, Fungicide, Rodenticide Act (FIFRA) must be reregistered under guidelines established by the Food Quality Protection Act (FQPA) to address data gaps that may exist. Data required for pesticide registration include but are not limited to
- the potential that the compound may remain as residue in potable water, fish, shellfish, and crops irrigated with treated water;
- the environmental fate of the compound, or where it goes after application and what happens to it when it gets there;
- how the compound breaks down and what the breakdown products are;
- whether the compound is absorbed through the skin or other routes of entry by test animals;
- the acute (short-term) and chronic (long-term) toxicity of the compound to test animals;
- whether the compound causes birth defects, tumors, or other abnormalities in test animals after long-term exposure; and
- the toxicity of the compound to aquatic organisms such as waterfowl, fish, and invertebrates.
Based upon registration data, residue tolerances are set by dividing the amount of residue that causes no observable effect to chronically exposed test animals by 100 or 1000 and estimating how much residue can be allowed in a commodity so that an average-sized person would ingest or come in contact with less than that amount.
Based upon tolerances, residue data, and environmental fate, water-use restrictions or precautions for drinking, swimming, fish consumption, irrigation, and watering livestock are placed on the label. Some compounds such as copper and glyphosate have no use restrictions at labeled use rates, while others have various restrictions on certain uses. Read the label carefully to determine the water-use restrictions for every aquatic herbicide you apply.
All herbicide containers must have attached to them a label that provides instructions for storage and disposal, uses of the product, and precautions for the user and the environment. The label is the law. It is unlawful to alter, detach, or destroy the label. It is unlawful to use an herbicide in a manner that is inconsistent with or not specified on the label. Note that aquatic weeds that are not specified on the label may be treated, and application methods not mentioned on the label may be used as long as they are not prohibited on the label. It is unlawful to transfer a herbicide to an improperly labeled container. Misuse of a herbicide is a violation of federal and state law. Herbicides used in water contrary to label directions may make water unfit for fishing, irrigation, swimming, or domestic use.
Each herbicide contains a signal word on the label, either "CAUTION," "WARNING," or "DANGER"1. The signal words indicate the acute toxicity of the compound to the person applying the herbicide, with "CAUTION" indicating the least toxic and "DANGER" indicating the most toxic. Some of the aquatic herbicides are quite toxic in the concentrated form and special care must be taken when handling these products. The label contains valuable information on personal protective equipment for the use of each aquatic herbicide. A "CAUTION" on the label does not necessarily indicate that the compound is relatively safe in the aquatic environment, however. Read and understand the entire label to protect your health and the health of people, plants, and animals that use this water.
The herbicide label contains a great deal of information about the product and should be read thoroughly and carefully before each use. Before applying a herbicide, read the label to determine the following:
- Is the product labeled for the site, i.e., ditch banks only, canal banks, ponds, lakes, rivers, etc.?
- Can the target weed be controlled with the product?
- Can the herbicide be used safely under the current application conditions?
- How much herbicide is needed?
- What restrictions apply to watering livestock, fishing, swimming, consuming potable water, and irrigation?
- What is the toxicity of the product to fish and non-target vegetation?
- When should the herbicide be applied (time of year, stage of plant growth, etc.)?
- Is the herbicide classified restricted use?
- What is the signal word (DANGER, WARNING, CAUTION1)?
- What safety equipment should be worn?
1 The signal words DANGER, WARNING, or CAUTION included on each herbicide container denote the relative toxicity of the concentrated product in the container. A DANGER signal word indicates the concentrated product is highly toxic via exposure routes such as ingestion or dermal exposure. A WARNING signal word indicates that the product may result in acute illness due to ingestion or dermal exposure. A CAUTION signal word indicates the product is slightly toxic or relatively non-toxic. These terms apply to the concentrated product in the container and its potential to harm the handler. They do not refer to the toxicity of the product once it has been applied to the water.
Herbicide Use Rates
To work as intended, herbicides must be applied according to the use rates recommended on the herbicide label for a given target plant. Simply to dump an unmeasured amount of an herbicide compound into the water column would certainly be environmentally and fiscally irresponsible, and it might be ineffective as well. Before they purchase herbicide, aquatic managers must "do the math." The first step is to measure the area and volume of water where the target plant is growing. Next, read the label to determine the parts per million (ppm) or parts per billion (ppb) of the herbicide necessary to control the nuisance plant. Finally, calculate how much herbicide will be necessary to achieve the goal.
For example, an applicator by the name of Sandy Trails wants to treat a 10-acre area with an average depth of 6 feet. Sandy calculates this as 60 acre-feet (10 acres x 6 feet deep). The amount of water in this 60-acre-foot area comes to 19.58 million gallons. In order to achieve the label-recommended target concentration of the herbicide endothall of 3 ppm, Sandy would need to apply 115 gallons of product to the 10-acre area (11.5 gallons per acre). Now Sandy knows exactly how much herbicide to buy.
Application equipment is calibrated to deliver a known concentration of herbicide as the boat makes numerous passes within the treatment area. For emergent plant control, the use recommendations are very similar to those for terrestrial agriculture. A typical emergent application will be in the range of 1 quart to 2 gallons of product per acre, and the objective is for the vast majority of the herbicide to fall on the emerged portions of the plant. Inevitably a small amount of herbicide will hit the water instead of the plants, and this is why certain herbicides such as glyphosate and imazapyr, which are for emergent plant control only, are specially labeled for either aquatic or terrestrial use. Only herbicide preparations with an aquatic label will be effective when used on aquatic plants. Residues of emergent-plant-control herbicides in the water are in very low concentrations that will not typically impact submersed vegetation.
Contact Herbicides
Contact herbicides act quickly and are generally lethal to all plant cells with which they come in contact. Contact herbicides do not move extensively within susceptible plants: they kill only the part of the plant they touch. For this reason, they are generally more effective on annual plants (plants that complete their life cycle in a single year) or smaller perennial plants (plants that persist from year to year). Large perennial plants can be defoliated by contact herbicides, but it is extremely difficult to contact all of the plant parts (think of a dense cattail stand with plants growing 8 to 10 feet tall) and perennials often resprout from unaffected plant parts and rhizomes growing in the substrate. Submersed aquatic plants that come into contact with sufficient concentrations of the herbicide in the water for sufficiently long periods of time will be affected, but they often regrow from unaffected parts, especially roots and rhizomes that are protected beneath the sediment. Because the entire plant is not always killed by contact herbicides, retreatment is often necessary, sometimes two or three times per year. Endothall, carfentrazone, diquat, flumioxazin and copper are contact aquatic herbicides.
Systemic Herbicides
Systemic herbicides are absorbed into the living portion of the plant and move within the plant. Different systemic herbicides are absorbed to varying degrees by different plant parts. Systemic herbicides that are absorbed by plant roots are referred to as soil-active herbicides, and those that are absorbed by leaves are referred to as foliar-active herbicides (Imazapyr is the only soil-active aquatic herbicide, and it is not applied as a pre-emergent herbicide for aquatic use). Other systemic herbicides, such as glyphosate, are only active when applied to and absorbed by the foliage. Triclopyr, 2,4-D, imazamox, imazapyr, fluridone, bispyribac, and glyphosate are systemic aquatic herbicides.
When applied correctly, systemic herbicides act slowly in comparison to contact herbicides. They must move to the part of the plant where their site of action is located. Systemic herbicides are generally more effective than contact herbicides for controlling perennial and woody plants. Some systemic herbicides are inherently selective (e.g., 2,4-D, triclopyr), while others are more broad-spectrum (glyphosate, imazapyr).
Broad-Spectrum Herbicides

Credit: Mark Hoyer, UF/IFAS
Broad-spectrum (sometimes referred to as nonselective) herbicides are those used to control all or most vegetation or a broad range of plant species. This type of herbicide is often used for total vegetation control in equipment yards and electrical substations and along banks of aquaculture ponds—any area where bare ground is preferred. Glyphosate is an example of a broad-spectrum aquatic herbicide. Diquat, endothall, and fluridone can be used as broad-spectrum aquatic herbicides but can also be used selectively under certain circumstances that will be discussed later in this publication.
While many herbicides such as glyphosate or diquat naturally control a broad range of plant species, application techniques often allow these products to be used selectively. Concentrated glyphosate can be placed directly on large woody species, and diquat spray can be directed on small patches of waterlettuce without causing widespread harm to nearby beneficial vegetation.
Selective Herbicides
Selective herbicides kill some species and have limited impact on others. A good example of a selective aquatic herbicide is 2,4-D, which can be used to control water hyacinth with minimal harm to maidencane or eel grass. Herbicide selectivity is based upon the relative susceptibility or response of a plant to a given herbicide. Many related physical and biological factors can contribute to a plant's susceptibility to an herbicide. Physical factors that contribute to selectivity include herbicide placement, formulation, and rate of application. Biological factors that affect herbicide selectivity include physiological factors, morphological factors, and stage of plant growth. A large percentage of aquatic herbicide treatments are applied with selective control in mind. Application can be selective simply by carefully placing the herbicide on target plants and avoiding non-target plants. For example, when small amounts of water hyacinth are growing among bulrush, an experienced applicator, using a type of applicator called a handgun, can control water hyacinth with 2,4-D and minimize impact to the bulrush community. Although diquat is a broad-spectrum herbicide, it is also a contact herbicide, so it will affect only the bulrush stems that are above the water surface and of those only the stems it contacts directly. The extensive underground rhizomes and roots will not be affected and the plant will regrow quickly after the initial effect of the herbicide. This is an example of selective weed control by herbicide placement.
Selectivity can also be affected by the amount of herbicide applied. For example, water hyacinth is selectively controlled among spatterdock (i.e., cow lily) using the recommended rate of 2,4-D for water hyacinth, but spatterdock can be controlled by using higher rates and granular formulations.
An herbicide must either be absorbed directly into the cells it contacts and kill those cells, or it must translocate (move through the plant) to the site where it is active. Herbicides may be bound on the outside of some plants or bound immediately after they enter the living part of the plant, so that they cannot move to their site of activity. For reasons that are not yet well understood, some selective herbicides translocate more readily in some plants than in others, and it is this phenomenon that causes their selectivity. Additionally, some plants have the ability to alter or metabolize an herbicide once it enters the plant, so that it no longer has herbicidal activity. Some herbicides affect very specific biochemical pathways in plants and are therefore selective only against a particular group or a few groups of plants because they are the only ones that have that particular pathway.
The physiology of perennial plants changes during the annual growth cycle, and their susceptibility to various herbicides changes in tandem with their physiology. During early stages of growth, for instance, when food reserves and other plant compounds are transported upward in the plant, it will be vulnerable to soil-active herbicides. (Although we don't typically use soil-active herbicides in aquatic plant control, we do get some activity with imazapyr.) Soil-active herbicides, then, will function best in the spring, whereas foliar-active herbicides like glyphosate are most active on perennials in the fall of the year, when the plants are translocating sugars from leaves to storage structures such as roots or rhizomes.
Environmental Considerations
Aquatic communities consist of aquatic plants including macrophytes (large plants) and phytoplankton (free-floating algae), invertebrate animals (such as insects and clams), fish, birds, and mammals (such as muskrats, otters, and manatees). All of these organisms are interrelated in the community. To survive, organisms in the community require a certain set of physical and chemical conditions to meet their needs for nutrients, oxygen, light, and space—and different populations of organisms depend on one another for these essentials. Aquatic weed control operations that are too drastic or not well planned can decimate or even eliminate populations of organisms in the community. Those losses to the community may have direct detrimental effects on other organisms or they may change the water chemistry in ways that harm other organisms.
Aquatic Plants
Aquatic plants are a natural and important component of aquatic communities (Section 1). They provide food for other aquatic organisms by fixing the sun's energy through the process of photosynthesis. Small invertebrate animals consume aquatic plants, periphyton (algae growing on larger plants), and phytoplankton, and are themselves consumed by larger animals such as birds or fish. Aquatic plants provide habitat for the small animals fish eat, and they provide protective cover for fish. Plants also provide nesting sites and food for birds and mammals. In addition, aquatic plants can improve the appearance of a water body. However, water is often naturally rich enough in the plant nutrients nitrogen and phosphorus for aquatic plants to grow so vigorously that they become a nuisance. They can hinder recreational use of water bodies or create flooding hazards by impeding drainage, which is often vital to low-lying residential communities. This is especially true for hydrilla, water hyacinth, alligator-weed, and Eurasian watermilfoil, which are non-native and invasive plants.
Although it is sometimes necessary to manage native aquatic plants, the majority of publicly funded aquatic plant management programs in Florida are aimed at hydrilla, water hyacinth, water lettuce, and torpedograss (alligatorweed is mainly controlled by the flea beetle). The reason for the concentration on invasives is that these non-native plants compete with native plants and grow well in Florida's warm climate. This combination reduces populations of desirable fish, decreases water quality, and hinders water use. If used imprudently, the herbicides that manage these non-native aquatic plants can harm native aquatic vegetation, but a careful management plan incorporating appropriate application rates, timing, and application techniques of aquatic herbicides can control non-native weeds with minimal impact on native plant populations. With good planning, it's possible to maintain a beneficial aquatic plant community for fish and wildlife habitat and many recreational uses. It is not always possible, however, to satisfy the demands of all water users. In some cases tradeoffs must be made. For example, it may not be possible to manage aquatic plants in a shallow eutrophic lake for fish habitat, waterfowl habitat, and water skiing all at the same time.
Aquatic plant control operations can have an indirect impact on phytoplankton. When lake managers use herbicides or grass carp to control large amounts of aquatic vegetation in a lake (>30% area covered with aquatic plants; see Section 1 for more information), nitrogen and phosphorus, plant nutrients that phytoplankton need to grow, are released into the water. The release of these nutrients spurs more phytoplankton growth in the lake, which turns the water green and decreases its clarity (Figure 5 on page 25).
Effects on Fish and Other Organisms
When used according to the label specifications, currently available aquatic herbicides are not toxic to fish, birds, or other aquatic organisms. They are also short-lived in the environment and do not accumulate in organisms. Environmental conditions are not always predictable, however, and under certain circumstances, fish kills can occur, usually as an indirect result of aquatic herbicide applications.
Fish kills are only likely to occur as a direct effect of herbicide application if an herbicide formulation known to be toxic to fish, such as the amine salt of endothall, is applied in an enclosed water body. The concentration of copper that is used for most herbicide applications is below toxic concentrations. However, rates recommended for difficult-to-control filamentous algae can be toxic to fish in enclosed ponds, and care should be taken when making this type of application. The greatest concern for copper toxicity is in low-alkalinity water, because the toxicity of copper to fish and many invertebrates (e.g., crayfish) increases as the alkalinity of water decreases (Table 3). This is especially true for most trout species. Most aquatic herbicides have very low toxicity to fish, and the concentration that occurs after application of recommended rates is less than concentrations that are toxic to fish (Table 4).
The most common reason for fish kills due to aquatic herbicide application is the indirect effect of lowered dissolved oxygen (DO) in the water. DO in lakes and ponds commonly ranges between 5 and 12 ppm (mg/liter). Aquatic plants and algae produce oxygen during the day via photosynthesis. Plants, algae, and animals consume oxygen throughout the day and night. Lowest concentrations occur during early morning hours, because aquatic plants consume oxygen during the night but do not produce oxygen because of the lack of sunlight. Fish populations can usually withstand the everyday fluctuations of DO, but many types of fish cannot tolerate prolonged periods of low DO. Natural fish kills can also occur in highly productive waters when phytoplankton populations die and cease producing oxygen after prolonged cloudy, still, warm weather.
When large numbers of aquatic plants are killed by an herbicide application, the decaying vegetation and lack of oxygen production may cause DO to become so low that fish cannot survive in the water and a fish kill occurs. Herbicides that are effective on higher plants and not phytoplankton minimize the potential for a fish kill because phytoplankton will continue to produce oxygen. Also, the danger of fish kills is lower in cooler water because it can hold more oxygen than warm water and bacterial decay of the dead vegetation is slower. Herbicide applications to large weed populations in warm water during periods of prolonged still and cloudy weather and where fish movement is restricted should be avoided to minimize the potential for fish kills. Large weed populations should be brought under control gradually by a series of applications to portions of the water body. Large populations should be treated during the spring, when water temperatures are lower. Once under control, weeds should be maintained at low densities.
Herbicide-related fish kills, either direct or indirect, are not likely to occur as a result of partial-area applications in large water bodies because fish can sense low DO and swim to more oxygen-rich water. When making partial applications of herbicides such as using the diethylalkylamine salt of endothall, which can be toxic to fish at recommended use rates, applications should be started near shore and should proceed toward open water. This allows fish to escape to untreated water. Take all possible precautions when applying aquatic herbicides to avoid conditions that can lead to fish kills.
Credit: UF/IFAS Center for Aquatic and Invasive Plants
Fate of Aquatic Herbicides in the Environment
The concentration of herbicide in water immediately after proper application of aquatic herbicides for submersed weed control is very low (Table 5). For example, when 2 gallons of diquat are applied to an acre of 6-foot-deep water, the nominal concentration is 0.0.25 ppm. Lower herbicide concentrations in water result from foliar applications to floating or emergent plants because the herbicide is directed onto the plants and very little herbicide reaches the water.
Herbicide residues are subject to dispersion, dilution, sorption, uptake, and degradation in the aquatic environment. Dispersion is the movement of herbicide residues outside of the treatment zone. It leads to dilution of the residues to a lower concentration. Dispersion and dilution are major processes affecting herbicide concentrations when smaller areas of larger water bodies are treated. Sorption is the binding of herbicide residues to particulate matter (e.g., clay and suspended organic matter) or to ions in the water. Diquat residues, for instance, are rapidly bound and inactivated by adsorption to clay or suspended organic matter, while glyphosate residues are inactivated by ionic bonding to cations (positively-charged particles such as calcium and magnesium) in the water column. Both adsorption and ionic bonding are examples of sorption. For emergent treatments, plants account for a large fraction of herbicide uptake while submersed plant uptake accounts for only a small fraction of the herbicide applied. Degradation is the ultimate fate of the herbicide molecule. Herbicides are degraded via processes such as hydrolysis (broken down by water) (for instance, in the case of carfentrazone), microbial activity (endothall), and photolysis (broken down by light) (fluridone and imazapyr). Compounds such as diquat and glyphosate are rapidly inactivated by sorption, and then slowly degraded via microbial processes. Both dispersion and degradation affect the fate of herbicides in the environment. Understanding how the environment affects herbicides is key to effective treatment. Even in cases where dissipation is slow and the herbicide remains in the treated area for a long time, the herbicide may yet be ineffective if it has been made biologically unavailable by adsorption to bottom sediments or any of the several processes described above.
Aquatic herbicides are non-persistent in treated water, which means that they disappear rapidly. Herbicide half-lives are shortest when spot treatments are made in large bodies of water because of dilution. Aquatic herbicides are water soluble and quickly dilute to non-detectable concentrations. Residues decline at different rates and by different methods. Table 5 lists rates of breakdown and major routes of degradation of aquatic herbicides. Because of environmental factors, rates of degradation are often much faster than those listed in Table 5, and these values should be used only for comparison.
Diquat
When applied to enclosed ponds for submersed weed control, diquat is rarely found in a treated pond longer than 10 days after application and is often below detection levels 3 days after application. The most important reason for the rapid disappearance of diquat from water is that it is rapidly taken up by aquatic vegetation and bound tightly to particles in the water and bottom sediments. When bound to certain types of clay particles, diquat is not biologically available. When bound to organic matter, it is slowly degraded by microorganisms (bacteria). When diquat is applied foliarly (to plant leaves), it is degraded to some extent on the leaf surfaces by sunlight (called photodegradation). Finally, because diquat is bound in the plant tissue, as the tissue decays a proportion is probably degraded by microorganisms.
Endothall
Endothall is rapidly and completely broken down into naturally occurring compounds by microorganisms. The by-products of endothall dissipation are carbon dioxide and water. Complete breakdown usually occurs in about 2 weeks in water and 1 week in bottom sediments. The rate of degradation more rapid at higher temperatures.
Glyphosate
Glyphosate is not applied directly to water for weed control, but when it does enter the water, it forms ionic bonds with calcium, magnesium, and other cations, resulting in rapid deactivation. Microbes break glyphosate down into carbon dioxide, water, nitrogen, and phosphorus over a period of several months.
2,4-D
2,4-D photodegrades on leaf surfaces after foliar applications and is broken down by microbial degradation in the water and sediments. The speed of microbial degradation is directly related to air and water temperature. Complete decomposition usually takes about 3 weeks in water and can be as short as 1 week when the water and air are warm. 2,4-D breaks down into naturally occurring compounds. Two pounds of 2,4-D amine will break down into 1-pound carbon dioxide, 1/4 pound water, 1/4 pound ammonia, and 1/2 pound chlorine.
Fluridone
Dissipation of fluridone from water occurs mainly by photodegradation. Metabolism by tolerant organisms and microbial breakdown also occurs, and microbial degradation is probably the most important method of breakdown in bottom sediments. The rate of breakdown of fluridone is variable and may be related to time of application and water depth. Applications made in the fall or winter, when the sun's rays are less direct and days are shorter, result in longer half-lives. Residues tend to last longer in deeper water. Fluridone usually disappears from pond water after about 3 months, but can remain up to 9 months. It may remain in bottom sediment between 4 months and 1 year.
Flumioxazin
Flumioxazin is used for both submersed and emergent weed control. This molecule is degraded via pH-dependent hydrolysis. At a pH range of 8 to 9, the half-life of the product can be less than 1 hour. At a neutral pH, the half-life is approximately 24 hours, and at a pH of 5, flumioxazin has a half-life of 3 to 5 days. The pH of the water is the major determining factor in whether flumioxazin will be a good fit for a given site. Eventually microbes decompose and mineralize flumioxazin (which means that they break it down into simple inorganic compounds.)
Carfentrazone
Like flumioxazin, Carfentrazone is degraded via pH-dependent hydrolysis; the higher the pH, faster the rate of degradation. Degradation of the carfentrazone molecule can occur within 1 day in more alkaline waters and may occur over several days in lower-pH water bodies (those with a pH of 5.5 to 7). As with flumioxazin, microbial activity eventually results in mineralization of the metabolites of carfentrazone (metabolites are the molecules that remain after microbial decomposition).
Triclopyr
Triclopyr is used for both submersed and emergent plant control. In the water column, triclopyr is mainly degraded by photolysis (photolysis is the process by which chemical compounds are decomposed by photons: more simply, sunlight breaks them apart). Microbial activity is also an important process in the degradation of the triclopyr molecule. Rates of photolysis are dependent on water depth and clarity, and microbial activity is influenced by water temperature.
Imazapyr
Like glyphosate, imazapyr is not applied directly to water for weed control, but residues that do end up in the water are subject to photolysis and microbial degradation. Typical half-lives of imazapyr in the water column are in the range of 7 to 14 days depending on water depth and clarity. Imazapyr is very soluble in the water column, and high solubility does not result in strong binding.
Imazamox
Imazamox is applied for both submersed and emergent weed control. In lake water, it is broken down by photolysis and microbial degradation. Typical half-lives of imazamox in the water column are in the range of 7 to 14 days depending on water depth and clarity. Imazamox is highly water soluble and does not bind to sediments or to particulate organic matter.
Bispyribac
Bispyribac can be used for both submersed and emergent weed control. Most byspyribac in the water column and sediments is decomposed by microbes. Following whole-lake treatments, bispyribac half-lives have ranged from 30 to 90+ days. To date, the speed of microbial degradation of bispyribac has not been closely linked with the time of year or temperature. Given the long-term exposure requirements for bispyribac to control many submersed plants, the slow rate of degradation can be beneficial.
Copper
Copper is a naturally occurring element and essential at low concentrations for plant growth. It does not break down in the environment, but it forms insoluble compounds with other elements and is bound to charged particles in the water. It rapidly disappears from water after it is applied as an herbicide. Because it is not broken down, however, it can accumulate in bottom sediments after repeated high application rates. But copper accumulations rarely reach levels that are toxic to organisms or significantly above background concentrations in the sediment.
Penoxsulam
Photodegradation accounts for most of the dissipation of penoxsulam from water. Microbial degradation is probably the most important method of breakdown in bottom sediments. Penoxsulam breaks down at a variable rate; the speed of degradation may be related to time of the application and the depth of the water. Applications made in the fall or winter, when the sun's rays are less direct and days are shorter, result in longer half-lives, and residues tend to last longer in deeper water. Penoxsulam usually disappears from pond water after about 3 months, but it can remain up to 9 months.
Maintenance Control of Aquatic Weeds
Maintenance control (or management) is a strategy aimed at controlling invasive plants at low levels and before they reach a problem level (rather than attempting total eradication, which is often not feasible). Florida Statute FAS 369.22 defines maintenance control as follows:
...a maintenance program is a method for the control of non-indigenous aquatic plants in which control techniques are utilized in a coordinated manner on a continuous basis in order to maintain the plant population at the lowest feasible level as determined by the department [Department of Natural Resources]. FAS 369.22
Maintenance control of aquatic weeds reduces the detrimental environmental effects caused by the weeds and reduces the potential for environmental impacts from aquatic plant control activities. Maintenance control offers the following advantages:
- detrimental impacts of aquatic weeds on native plant populations are reduced;
- detrimental impacts of aquatic weeds on water quality are reduced;
- the amount of organic matter deposited on the lake bottom from natural processes is reduced;
- the amount of organic matter deposited on the lake bottom after control of aquatic plants is reduced; and
- less herbicide is used in the long term.
For example, maintenance of water hyacinth to less than 5% coverage under experimental conditions reduced herbicide usage by a factor as great as 2.6, reduced deposition of detritus by a factor of 4.0, and reduced depression of DO that occurred beneath the vegetation mats.
One difficulty lake managers sometimes encounter when conducting a maintenance control program is that people do not perceive a weed problem and therefore they question the need to spray. Public education is thus an important part of a successful maintenance control program. Maintenance management is the most environmentally sound method for managing water hyacinth, for instance. Unmanaged, water hyacinth can double every 7 to 10 days. Ten plants amounting to a pound or two in weight and a couple feet in area can grow in a single season to 200 tons covering a full acre of formerly clear, navigable water. With a little education, the benefit of controlling those 10 plants early will be obvious to concerned observers.
Maintenance management is not the solution for every lake plant problem. It works for water hyacinth, but is more difficult for submersed weeds such as hydrilla. In south Florida canals, maintenance management of hydrilla has been successfully implemented, but further research will be necessary to develop cost-effective programs for maintenance management of hydrilla in lakes. Once developed, maintenance management programs for hydrilla in lakes should provide more environmentally sound aquatic weed control. In northern lakes, cold weather, ice, and snow perform an annual natural maintenance management program. Aquatic plant management is often an annual affair but some evidence indicates that, when properly planned and applied, management during one growing season may carry over to the following growing season or beyond.
Credit: UF/IFAS Center for Aquatic and Invasive Plants
Manipulating Plant Communities
The aesthetics and fish and wildlife habitat values of lakes and reservoirs can sometimes be greatly enhanced by establishing and managing certain desirable aquatic plants. Many lakes have sparse vegetation, undesirable species, or plants growing in the wrong places.
Manipulating habitat (e.g., substrate type or lake bottom slopes), selectively removing undesirable plants or plants that occur in undesired locations, and planting desired plants in desirable locations are all ways of managing aquatic plants to improve the quality of a lake. Habitat manipulation techniques add options and versatility to an aquatic plant management plan.
Where it is legal, excavation can deepen aquatic environments to exclude plants from areas where they are not desired, and the substrate can be used to form shallows for planting desired aquatic plants. When manipulating habitat like this, it is extremely important to determine the low, average, and high water lines of the lake. While some wetland plants will tolerate dry and wet seasons, there are many that will die if they are kept too wet or too dry. Individual plant species also require different water depths to be successful. Successful designs create habitat of the proper depth for the desired plant species.
Some aquatic management techniques that control plants can also promote desirable species and improve habitat. The physical removal of problem aquatic plants can make room for desirable plants to grow. For instance, mechanical harvesting of water milfoil, sometimes stimulates wild celery to grow. If mechanical harvesting is not an option, the herbicide 2,4-D can be used to shift plant community composition, for instance, from watermilfoil and coontail to beneficial pondweeds and wild celery (Nichols 1986). Screens and harvesters can channelize plant beds to produce island habitats, increase edge, and form cruising lanes for boaters and gamefish. Aluminum sulfate (alum) can reduce algae and thus improve water clarity for larger plants to grow. These are only a few of the many methods available to promote desirable aquatic plant growth in lakes and reservoirs.
As a general rule, adding desirable plants to lakes creates the bigger benefit compared to removing undesirable plants, but decisions about plants to add must be made with special care. Section 1 shows, for instance, that different types of plants (e.g., emersed, submersed) and individual species within each plant type require different conditions to survive. For example, water shield is an excellent food source for waterfowl and a potential plant for re-vegetation of lakes with no aquatic plants, but it only thrives in acidic, softwater lakes (Hoyer et al. 1996). Therefore, attempts to plant water shield in alkaline, hardwater lakes would waste both money and effort. Before attempting to re-vegetate, the lake manager must learn the types and species of aquatic plants that will grow well in that particular water body.
Conclusion
Aquatic plant management is a vital element of the human endeavor to protect and preserve the environment. As the United States continues into the 21st century, there is widespread concern for the environment. This concern is certainly warranted, considering the large changes that have occurred to our planet since the turn of the 20th century. Some of these concerns, however, are based more on myth than on science. Scientists do not have all the answers, but our scientific knowledge is adequate enough to provide the guidance necessary to minimize environmental risks, while implementing an aquatic plant management program. Like all human endeavors, lake management plans can sometimes go astray when well-meaning people make decisions based on faulty information. The goal of UF/IFAS Florida lakewatch in this and other publications about lakes is to provide reliable information on lake and fisheries management that will contribute to the elimination of many of the myths that prevented effective management of our aquatic systems in the past. The ultimate success or failure of even the best management programs depends upon the people who decide to become involved becoming educated about the goal and the way to achieve it.
As more people use our lakes, more controversies about how the lakes should be managed will inevitably arise. Fortunately, an understanding of the history of aquatic plant management reveals that the problem is actually rather simple. One thing is clear: Although conflicts may seem diverse and unrelated, nearly all are rooted in conflicting values regarding what makes a quality lake and how lakes should be used. Value judgments are brought to the planning process not only by citizens, but by scientists and representatives from the federal, state, and local agencies charged with managing aquatic systems.
Florida LAKEWATCH has long suggested that conflicts be minimized through the development of comprehensive, integrative management plans for individual water bodies. This is hardly to suggest that the development of an aquatic plant or lake management plan is an easy task. Many management plans are either short-lived or dysfunctional when implemented because of disorganized citizen participation and disorganized input from the scientific community during the planning process. For example, the planning process can be drawn out over a long period of time (i.e., years) and the plan ultimately compromised by various stakeholders (e.g., regulatory agencies, homeowners, anglers, and business owners) unpredictably interjecting themselves into the process. The process is further complicated when these parties are supported by experts (e.g., academics, private professionals, or agency personnel) representing conflicting and seemingly irreconcilable opinions on technical issues.
Simon (1955) wrote that significant changes in human behavior can only be brought about rapidly if the persons who are expected to change participate in deciding what the changes shall be and how they shall be made. If we recognize the fundamental truth of Simon's statement, how then do we resolve conflict and develop comprehensive aquatic plant management programs, lake management programs, or water resource policy in a timely manner? The answer is that there is no surefire method. Search for the approach that is best suited for your community.
A new approach that attempts to improve upon traditional modes of public participation and scientific peer review in order to more efficiently integrate them with the policy making process is TEAM "Together for Environmental Assessment and Management: A process for Developing Effective Lake Management Plans or Water Resource Policy" (Canfield and Canfield 1994). TEAM's strength comes from combining in a new formula the most democratic attributes of public participation and scientific peer-review processes. TEAM provides citizens and professionals separate, but complementary, forums and responsibilities, unlike traditional approaches such as a task force or committee where lay citizens and professionals must work as a single unit. With the TEAM approach, citizens first identify and prioritize issues and potential courses of action that they believe are important. The experts then provide the citizens with a discussion, including pros and cons, of the technical issues relevant to the problems and potential courses of action identified. These complementary roles provide citizens with the technical information necessary to make informed choices. Another benefit of the TEAM approach is that it rescues experts from the inappropriate and sometimes awkward position of making policy judgments.
TEAM is designed to facilitate the development of an aquatic plant management or lake management plan reasonably quickly. TEAM ensures that the opinions of stakeholders as well as those unable to become involved because of limited time are fairly represented. Using teams of experts that discuss the pros and cons of each issue offers a structure for a debate of technical issues that will help identify points of agreement and disagreement, as well as areas where more information is needed. The TEAM approach permits experts' peers to judge the merits of their technical arguments, rather than forcing citizens or elected policy makers into the position of trying to become scientists. And importantly, TEAM, with the comprehensive participation of stakeholders, the busy public, and experts, is intended to minimize potential delays and/or litigation.
The end product of the TEAM approach or any other approach is a plan of action, the lake management plan. Lakes, ponds, reservoirs, and all other water bodies are dynamic, adaptable, and ever-changing ecosystems. Aquatic plant management plans or lake management plans must be just as dynamic and adaptable. The question of how best to manage aquatic plants, like so many environmental management questions, is often an ideological battleground, but usually not one on which a definitive "win" can be recorded by any one of often multiple "armies." Stakeholders may go into a lake-management conflict insisting that they won't bend on any issue, but eventually most people come to understand that compromise is necessary to move forward and develop a workable plan. Fortunately, history shows that careful, effective management plans can be developed that protect and preserve the nation's waters.

Credit: UF/IFAS Center for Aquatic and Invasive Plants
Literature Cited
Caffrey, A.J., M.V. Hoyer, and D.E. Canfield, Jr. 2007. "Factors affecting the maximum depth of colonization by submersed macrophytes in Florida lakes." Lake and Reservoir Management. 23: 287–297.
Canfield, D.E., Jr. and M.V. Hoyer. 1992. Aquatic macrophytes and their relation to the limnology of Florida lakes. Final report. Bureau of Aquatic Plant Management, Florida Department of Natural Resources, Tallahassee, Florida.
Canfield, S.L. and D.E. Canfield, Jr. 1994. "The TEAM approach, 'Together for environmental assessment and management': A process for developing lake management plans or water resource policy." Lake and Reservoir Management. 10: 203–212.
Capinera, J. and H. S. Walden. 2013. Rat lungworm Angiostrongylus cantonensis (Chen, 1935) (Nematoda: Strongylida: Metastrongylida). EENY570. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/in1007
Capinera, J. and J. White. 2011. "Terrestrial Snails Affecting Plants in Florida
Cole, G.A. 1983. Textbook of Limnology. Third Edition. Waveland Press, Inc. Illinois, Ohio.
Fasulo, T. R. 2011. Applesnails of Florida Pomacea spp. (Gastropoda: Ampullariidae). EENY323. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/in598
Gettys, L. A., W. T. Haller and M. Bellund, eds. 2009. Biology and control of aquatic plants: a best management practices handbook. Aquatic Ecosystem restoration Foundation, Marietta, GA. 210 pages.
Hoyer, M.V. and D.E. Canfield Jr. 1994. "Bird abundance and species richness on Florida lakes: Influence of trophic status, lake morphology, and aquatic macrophytes." Hydrobiologia. 297/280: 107–119.
Hoyer, M.V., D.E. Canfield Jr., C.A. Horsburgh, and K. Brown. 1996. Florida freshwater plants: a handbook of common aquatic plants in Florida lakes. SP 189. Gainesville: University of Florida Institute of Food and Agricultural Sciences.
Hoyer, M. V., C. A. Horsburgh, D. E. Canfield, Jr., and R. W. Bachmann. 2005. "Lake level and trophic state variables among a population of shallow Florida lakes and within individual lakes." Canadian Journal of Fisheries and Aquatic Sciences. 62: 1–10.
Hutchinson, G.E. 1975. A Treatise on limnology. Vol. III. Limnological Botony. John Wiley and Sons, New York, NY. 660 pp.
Nichols, S.A. 1986. "Community manipulation for macrophyte management." Lake and Reservoir Management. 2: 245–251.
Pearsall, W.H. 1920. "The aquatic vegetation of the English lakes." Journal of Ecology. 8: 163–201.
Pelikan, J., J. Svoboda, and J. Kvet. 1971. "Relationship between the population of muskrats (Ondatra zibethica) and the primary production of cattail (Typha latifolia)." Hydrobiologia. 12: 177–180.
Simon, H.A. 1955. "Recent advances in organization theory." In S. K. Bailey, et al. Research frontiers in politics and government. Brookings Institution, Washington, DC.
Smith, L.M. and J.A. Kadlec. 1985. "Fire and herbivory in a Great Salt Lake marsh." Ecology. 66: 259–265.
Wetzel, R.G. and R.A. Hough. 1983. "Productivity and role of aquatic macrophytes in lakes: an assessment." Polskie Archiwum Hydrobiologii. 20: 9–19.
UF/IFAS Florida LAKEWATCH
UF/IFAS Florida LAKEWATCH (FLW) is one of the largest citizen-based volunteer monitoring endeavors in the United States with more than 1,500 individuals monitoring more than 700 lakes and other bodies of water in more than 50 Florida counties. Staff from UF/IFAS's Program in Fisheries and Aquatic Sciences, School of Forest Resources and Conservation train volunteers throughout the state to conduct monthly long-term monitoring of both fresh and saline water bodies. Florida LAKEWATCH uses the long-term data to provide citizens, agencies, and researchers with scientifically sound water management information and educational outreach.
To become part of the Florida LAKEWATCH team, volunteers are required to have access to a boat and complete a two-hour training session. During the session, volunteers learn to collect water samples, take water clarity measurements, and prepare algae samples for laboratory analysis. Once a volunteer is certified by a regional coordinator and sampling sites are established, he or she will sample the designated stations once a month. Samples are frozen immediately upon being collected and are later delivered to a collection center, where they are stored until they can be picked up by Florida LAKEWATCH staff and delivered to the water chemistry laboratory at the UF/IFAS Program in Fisheries and Aquatic Sciences, School of Forest Resources and Conservation.
In return for participation, volunteers receive:
- personalized training in water monitoring techniques,
- use of lake sampling materials and water chemistry analysis,
- periodic data reports, including an annual data packet regarding their water body,
- invitations to meetings at which Florida LAKEWATCH staff provide an interpretation of the findings as well as general information about aquatic habitats and water management,
- access to freshwater and coastal marine experts, and
- free newsletter subscription and educational materials regarding lake ecology and water management.
For more information, contact:
UF/IFAS Florida LAKEWATCH
UF/IFAS School of Forest, Fisheries, and Geomatics Sciences, Program in Fisheries and Aquatic Sciences
7922 NW 71st Street
Gainesville, FL 32653-3071
Phone: (352) 392-4817
Toll-free: 1-800-LAKEWATCH (1-800-525- 3928)
E-mail: lakewatch@ufl.edu
Website: https://lakewatch.ifas.ufl.edu/
Impact of Varying Aquatic Macrophyte Abundances on Some Lake Uses (-), problematic (+), beneficial (-,+), both problematic and beneficial depending on circumstances.