Introduction
The aquaculture of hard clams (Mercenaria mercenaria) in Florida has grown rapidly over the last two decades. Hard clams have notably few infectious diseases, compared to other bivalve molluscs, and to date no significant problems due to infectious diseases have been observed in cultured clams from Florida waters. There is a growing concern, however, that disease- causing agents may appear as production densities increase. Information provided in this document is intended to familiarize clam growers with common clam diseases.
Gross Signs of Disease in Hard Clams
Gross signs of infectious disease in juvenile or adult hard clams may go unnoticed because clams are infaunal; that is, living buried in the sediment. However, most diseased or stressed individuals will rise to the sediment surface. Additional signs of infectious disease in clams may include: gaping (inability to hold the valves closed); shell deformities or chipping of the shell margin; deposits or blisters on the inner surfaces of shells; excess mucus production; watery meats; dark, pale, or discolored meats; lesions or ulcers of the mantle, adductor muscle, or foot; or retracted and/or swollen mantle edges. These signs are not necessarily indications of infectious disease; they may also be associated with noninfectious diseases and adverse environmental conditions.
Types of Clam Diseases and Pests
Pathogens can potentially infect all life stages of hard clams. Organisms of particular concern include QPX (Quahog Parasite Unknown), which has caused significant mortality of cultured clams in northeastern states, and Perkinsus spp., an oyster disease which clams are known to carry, though they do not get sick. Other potential pathogens of M. mercenaria include common bacteria in the environment, such as Chlamydiales and Rickettsiales. It should be noted that none of these diseases affect humans.
QPX
QPX, short for Quahog Parasite Unknown, is the only significant pathogen of hard clams. Significant prevalence of QPX, a "slime-net" protist, has been associated with clam mortality (up to 95%) from Canada to Virginia. QPX has not been detected in seed clams, suggesting that clams become infected in the planting areas. Observable signs of QPX include a retracted, swollen mantle edge and visible nodules in the tissue. Stained tissue sections are typically used to identify the QPX organism, which ranges from about 2 to 20 microns in diameter. A characteristic feature is a clear, "halo"-like area surrounding the parasite (Figure 1). Moderate to severe infections result in slower growth and poor quality meat. Environmental factors contributing to the disease are still being clarified. QPX is apparently only found at high salinity sites (> 30 ppt). Mortalities have been primarily associated with additional stressors, including unusually low temperatures in northern states and very high-density culture.
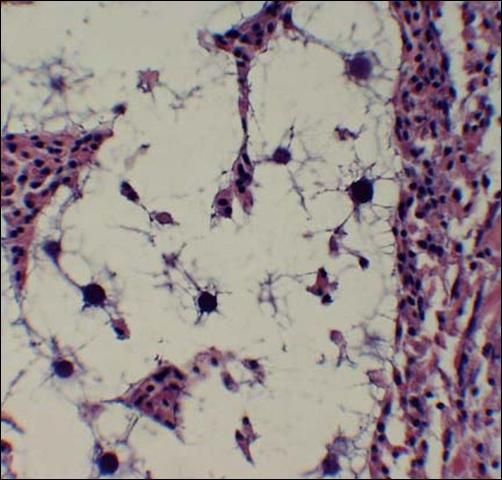 ;
;
Credit: R. Smolowitz.
Perkinsus spp. (Dermo)
Perkinsus spp. are protozoans that cause Dermo disease in eastern oysters, resulting in significant mortalities along the Atlantic and Gulf coasts. Although Perkinsus spp. are sometimes found in hard clams, they apparently do not cause noticeable signs of disease in clams. In oysters, signs of Dermo disease include an increase in pigmented cells in the heart, occlusion of blood vessels, and ulcers of the mantle. High levels of infection result in cessation of growth, poor meat quality, and loss of adductor muscle strength Perkinsus spp. are detected using Rays fluid thioglycolate medium (RFTM). Using this diagnostic technique, pieces of bivalve tissue are incubated in RFTM, allowing the parasites to enlarge. After several days, the tissue is removed from the medium and stained. The enlarged and stained parasites can then be easily viewed under a microscope (Figure 2). Environmental factors contributing to the disease include high temperatures (>25°C) and moderate salinities (~ 13-28 ppt). The parasite appears intolerant of salinities below 9 ppt. Parasitism by blood-sucking snails and the presence of environmental contaminants may be contributing factors.

Credit: Jamie Holloway, 2003.
Chlamydiales and Rickettsiales
Chlamydiales and Rickettsiales are intracellular bacteria that are relatively common in bivalve molluscs. Although these parasites can cause localized damage to gills and epithelia, and infrequent mortalities, they are generally considered benign. Chlamydiales-like organisms (CLO) and Rickettsiales-like organisms (RLO) are identified by examining tissue sections for swollen cells filled with inclusion bodies, which result as the organisms proliferate (Figure 3). Ruptured cells and tissue damage may also be observed. CLOs are usually found in the epithelium of the digestive gland, while RLOs are typically found in the gills. Both organisms are found more frequently in cultured bivalves than in wild stocks. Increased prevalence may be related to ease of transmission or increased stress on the host in high-density culture.
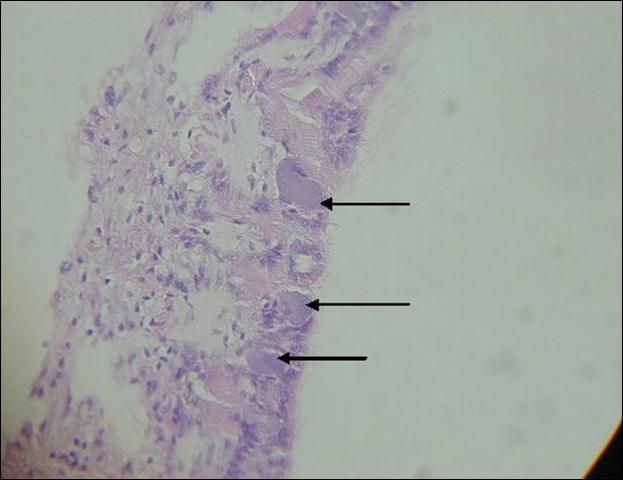
Credit: Denise Petty. 2005.
Pest Metazoans and Symbionts
Many marine organisms are occasional parasites or symbionts of clams. These can include parasitic nematodes (roundworms), cestodes (tapeworms), trematodes (flatworms), and copepods, which primarily enter clams through the walls of their digestive tracts. In addition, nemertean worms may be found as symbionts in mantle cavities. Usually, there are too few organisms to cause pathology or mortality. However, their presence in tissues may result in granulomas.
Granulomas
Granulomas are areas of thickened (fibrotic) tissue resulting from the clam's immune response to a foreign body, including any of the parasites listed above (Figure 4). Blood cells called granulocytes, which are part of the clam's immune system, migrate to and infiltrate the area surrounding the parasite (inflammation—tissue response to injury or infection of tissues, which serves to destroy, dilute, or wall off the injurious agent and the injured tissues). These cells may eventually encapsulate the parasite, forming an inflammatory lesion, or nodule, called a granuloma. Granulomas are detected by examination of tissue sections. The number of granulomas is usually too low to cause impairment of function, but if present in greater numbers, causative agents should be identified or ruled out, and further investigation may be warranted.
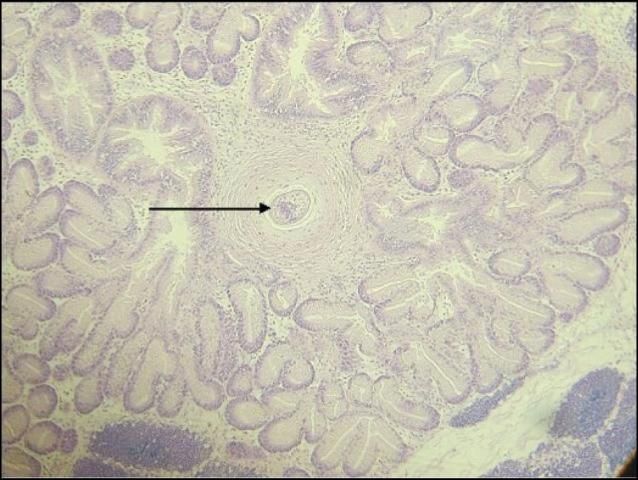
Credit: Denise Petty. 2005.
Significance in Florida
Florida cultured clams were examined for disease in February (winter) and August (summer) of 2003 using histology and RFTM. Three areas were monitored to represent the majority of clam production areas in Florida; northwest coast, east coast, and southwest coast. The Gulf Jackson High-Density Lease Area (HDLA), located in the inshore waters of the Gulf of Mexico, near Cedar Key, FL was sampled in both winter and summer. The Indian River Aquaculture Use Zone located in the Indian River, near Sebastian, FL, was also sampled in both winter and summer. Southwest Florida samples were taken at two different sites. Winter collections were taken from the Sandfly Key HDLA, near Placida, FL. Summer collections were taken from the South Pine Island HDLA, located in Pine Island Sound, near St. James, FL. Clams were collected by participating growers from culture bags that were being harvested for market. Live clams were shipped to the UF/IFAS Department of Fisheries and Aquatic Sciences, in Gainesville, FL. Samples of 60 clams, each, were divided equally between preparation for histology and RFTM.
In the group of clams examined, granulomas were the most frequently observed abnormality, followed by Rickettsiales-like organisms, non-granulomatous inflammation, and metazoans (Table 1).
There was no evidence of QPX in cultured clams from the three areas that were examined as part of this study. While QPX has never been identified in Florida clams, there is concern that it could be introduced to the state. Consequently, imported clam and oyster seed must be tested and proven free of the QPX agent before entry into Florida, as per Florida Department of Agriculture and Consumer Services Division of Aquaculture best management practices, Rule 5L-3.004, F.A.C. (https://www.fdacs.gov/Agriculture-Industry/Aquaculture/Aquaculture-Best-Management-Practices).
Perkinsus was identified in clams from all three study sites tested during this study. With the exception of one animal collected in the Southwest Florida area, all Perkinsus positive clams were collected during the summer sampling period. In total, 23% of the clams collected at Gulf Jackson, 7% from Indian River, and 67% from southwest Florida were affected. When Perkinsus is seen in clams, it is indicative of an adjacent population of infected oysters. Oysters are susceptible to infection by P. marinus, and there are many strains varying in infectivity. Therefore, although hard clams do not develop disease from Perkinsus infection, they can serve as vectors for the organism. Moving infected clams from one geographic location to another may introduce a new strain of Perkinsus to oysters and other susceptible bivalve molluscs.
Rickettsiales-like organisms were observed in the gills of clams collected in the winter from all three areas; 33% to 60% of the clams sampled had RLOs, with the greatest prevalence at Gulf Jackson. In the summer, only clams from the Indian River had RLOs (13%). Where RLOs were present, adjacent branchitis (gill inflammation) was infrequently observed. It is unclear if the RLOs are a significant problem for the clams no Chlamydiales-like organisms were observed in any of the clam samples.
Granulomas were the most frequently observed abnormality in the examined clams. Southwest Florida clams, regardless of season, were more affected than clams from the other collection areas. However, clams from all sites and seasons were affected. Prevalence of granulomas ranged from 13% (Gulf Jackson winter collection) to 53% (summer Southwest Florida collection). Metazoans, usually trematodes, were observed in some of the granulomas; up to 26% of the clams from a single collection were affected.
Non-granulomatous inflammation is a cellular response by an animal to a non-specific (unknown cause) injury. Non-granulomatous inflammation was observed in 66% of the winter-collected clams from the Southwest Florida area, with much less observed in clams collected in other sampling sites and seasons (less than 20%). The source(s) of the non-granulomatous inflammation was not determined.
Summary
To date, no significant problems due to infectious diseases have been identified in cultured clams from specific locations in Florida waters using histology or RFTM. Clinical disease caused by infectious disease was also not observed during the survey period. Our survey, therefore, suggests that, at present, there are no serious disease-causing agents associated with clams at the locations we examined. Although Perkinsus spp. was found, sometimes in relatively high numbers, it is a disease of oysters and has not been associated with clinical disease in hard clams. QPX, the clam pathogen of most concern, was not found. Granulomas, Rickettsiales-like organisms, non-granulomatous inflammation, and metazoans were found to varying degrees. In general, these were considered incidental and not significant; numbers were too low to cause pathology or mortality.
Most of these potential disease-causing organisms are present in the environment and are therefore difficult to control. Pathology may be exacerbated by stressors such as high temperatures (> 30°C), poor water quality (presence of noxious algae or contaminants, low dissolved oxygen, etc), or high stocking densities. There is a growing concern that the tendency to increase production and standing crop is conducive to the accidental introduction of disease-causing agents or increased stresses that may facilitate disease by these typically opportunistic organisms.
Shellfish culture in Florida is dependent on very few species. This increases the risk for catastrophic loss following the appearance of a new disease-causing agent. To minimize risk and potential economic impact on the industry, growers should be aware of potential shellfish diseases and be prepared to properly assess any occurrences of diseases. Assistance from UF/IFAS Extension shellfish and aquatic animal health specialists is available.
State Specialists
Primary Contact/UF/IFAS Extension Shellfish agent:
Cedar Key:
Leslie Sturmer
UF/IFAS Extension Shellfish Agent
(352) 543-5057
Aquaculture Extension veterinarians:
Gainesville:
Ruth Francis-Floyd, DVM
UF/IFAS Extension Veterinarian
386-643-8904
Tampa/Ruskin:
Roy Yanong, VMD
UF/IFAS Extension Aquaculture Veterinarian
UF/IFAS Tropical Aquaculture Laboratory
(813) 671-5230 Ext. 104
Glossary of Terms Used
Adductor muscle–The muscle that holds the two shells (valves) of a bivalve together
Bacteria–A microorganism that may cause disease
Branchitis–Inflammation of the gills
Clinical disease–Disease identified by direct observation of signs
Dermo–A disease of oysters associated with the protozoan Perkinsus marinus
Disease–Impairment of normal structure or function
Epithelium–Cells covering the internal and external body surfaces
Granuloma–Areas of thickened (fibrotic) tissue resulting from the organism's own immune response to a foreign body
Histology–Study of the minute structure, composition and function of tissues
Inclusion bodies–Abnormal structure within a cell
Infaunal–Organisms that live within the sediment
Infection–Invasion and multiplication of microorganisms in body tissues, especially those that cause local cellular damage
Infectious disease–Disease caused by a pathogen
Inflammation–Tissue response to injury or infection of tissues, which serves to destroy, dilute, or wall off the injurious agent and the injured tissues
Lesion–Visible disruption of the normal structure of a tissue caused by injury or disease
Mantle–Folds of the body wall that form a skirt around the body, terminating in an edge, the mantle margin
Mantle cavity–The space formed by the mantle, which encloses a large water space, the gills, and the body
Metazoa–Multicellular animals
Parasite–Animal that lives upon or within another living organism and obtains some advantage from it. There is usually some cost to the host
Pathogen–Any disease producing agent or microorganism
Pathology–The abnormal condition that constitutes disease
Prevalence–The total number of cases of a specific disease in a given population at a certain time
Protista–All single-celled organisms including bacteria, algae, slime molds, fungi and protozoa
Protozoa–A group of the simplest single-celled animals
QPX–A disease of hard clams associated with the protist Quahog Parasite X
RFTM–Rays fluid thioglycolate medium, used to detect Perkinsus, the agent of Dermo disease
Signs–Objective evidence of disease
Symbiont–An organism living in close association with a dissimilar organism, which may or may not gain an advantage from the relationship
Transmission–The transfer of disease from one organism to another
Vector–Carrier of an infectious agent, capable of transmitting infection from one host to another
Further Reading
Bower, S.M. and S.E. McGladdery. 2010. Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish. https://www.dfo-mpo.gc.ca/science/aah-saa/diseases-maladies/index-eng.html
Colson, S., and L. Sturmer. 2000. One shining moment known as Clamelot: The Cedar Key story. Journal of Shellfish Research 19(1): 477–480.
Couch, J.A. and J.W. Fournie. 1993. Pathobiology of Marine and Estuarine Organisms. Boca Raton, FL: CRC Press.
Dorlands Pocket Medical Dictionary. 1977. 22nd Ed. Philadelphia, PA: W.B. Saunders Company.
Kraeuter, J.N. and M. Castagna. 2001. Biology of the Hard Clam. Amsterdam, The Netherlands: Elsevier.
Meyers, T.R. 1981. Endemic diseases of cultured shellfish of Long Island New York, USA Adult and juvenile American oysters Crassostrea virginica and hard clams Mercenaria mercenaria. Aquaculture 22(4):305–330.
Ragone-Calvo, L.M., J.G. Walker, and E.M. Burreson. 1998. Prevalence and distribution of QPX, Quahog parasite unknown, in hard clams, Mercenaria mercenaria in Virginia, USA. Dis Aquat Org. 33(3)209–219.
World Organisation for Animal Health. 2021. Manual of Diagnostic Tests for Aquatic Animals. https://www.oie.int/en/what-we-do/standards/codes-and-manuals/aquatic-manual-online-access/