Nematodes in Field Corn Production
Nematodes are non-segmented roundworms that are generally microscopic. They live in animal hosts, soil/plant roots, or water. Nematodes in agricultural systems generally reside in soil and can be divided into three categories: (1) entomopathogenic nematodes that feed on insects; (2) free-living nematodes that feed on bacteria, fungi, or other nematodes and may be beneficial for crop production; and (3) plant-parasitic nematodes that feed only on plants and may drastically suppress crop yield.
Some of the more virulent plant-parasitic nematodes of corn reduce yield by decreasing root size and efficiency, leading to shorter or less-productive shoots. Infected plants may be reduced in size, show varying degrees of yellowing (chlorosis), and, in severe cases, die. Nematode infection can also increase incidence of fungal root diseases. The amount of damage nematodes cause is related to their population densities. The greater the density, the greater the damage.
In Florida, sting nematode (Belonolaimus spp.) is the most problematic nematode on corn because it has a very high damage potential. However, sting nematode (Figure 1) is limited to very sandy soils (80 percent or greater sand content) with minimal organic matter. Many other nematodes also parasitize corn. Needle (Longidorus breviannulatus, Figures 2 and 3), stubby-root (Trichodorus spp. and Paratrichodorus spp.), and lance (Hoplolaimus spp.) nematodes can be quite damaging, even at low densities (40 nematodes or less per 100 cm3 soil before planting). Stunt (Tylenchorhnchus spp.) and lesion (Pratylenchus spp.) nematodes only cause damage at higher densities (100 or more nematodes per 100 cm3 soil before planting). Some species of root-knot nematodes (Meloidogyne spp.) may also damage corn. While many of these nematodes cause the most damage in sandy soils, most can do well in a wide range of soil types. One exception is needle nematode, which only infests very sandy soils (90 percent or more sand), but can be highly damaging—if any needle nematodes are detected in the soil before planting, the crop is considered at risk for damage.
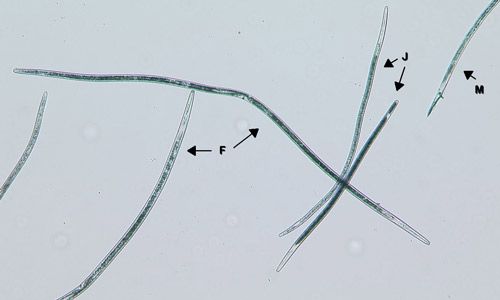
Credit: William Crow, UF/IFAS

Credit: Zane Grabau, UF/IFAS

Credit: Zane Grabau, UF/IFAS
Plant-parasitic nematode population densities can increase rapidly in corn fields. This is because most plant-parasitic nematodes of corn complete their life cycle (egg, four pre-adult juvenile stages, egg-producing adult) in about one month depending on the nematode species and environmental conditions, so nematodes may go through four or more generations in a single growing season. A single mature female nematode of some species, particularly root-knot nematodes, produces hundreds of eggs, contributing to rapid nematode increase.
Plant-parasitic nematodes of corn spend their entire life in soil or roots and can be classified by where they reside when feeding, which is important when sampling for nematodes. Ectoparasites (ecto means outside), including sting, needle, and stunt nematodes, spend their entire lives outside the roots. Only the head end or stylet—a needle-like mouthpart that all plant-parasitic nematodes possess—of an ectoparasite enters the roots when it feeds (Figure 4).
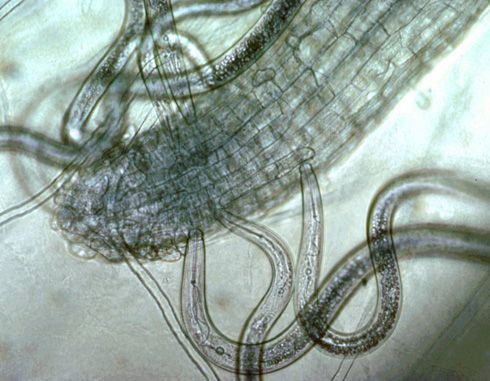
Credit: Ole Becker, University of California, Riverside. Used by permission
Endoparasites (endo means inside) enter the root as a juvenile or adult and remain in the root to feed. Some endoparasites, such as lesion nematode, are mobile while feeding (migratory endoparasite). Others, such as root-knot nematodes, do not move once they begin feeding (sedentary endoparasite). A portion of the population of endoparasitic nematodes can be found in the soil at any given time because eggs generally hatch in the soil, and mobile nematode stages may move freely in soil or from root to root. Stubby-root and lance nematodes may be either endoparasites or ectoparasites. See the UF/IFAS EDIS publications on sting nematode and stubby-root nematode for more information about these nematodes.
Sampling for Nematodes
Determining what nematodes are present in a field and at what densities is helpful for developing a plan for managing nematodes. This can be accomplished by submitting soil or root samples to a professional nematology diagnostic lab such as the University of Florida/IFAS Nematode Assay Laboratory. These samples may be submitted to aid with the diagnosis of disease problems or for advisory service to predict whether or not a potential nematode problem may exist for a future crop. This information allows growers the opportunity to implement management practices to reduce the damage that nematodes may cause.
Detailed information about sampling for nematodes and submitting samples can be found at the UF/IFAS nematode assay laboratory website. When submitting samples for testing for nematodes, either soil or soil-and-plant-root samples should be included. Always include soil because ectoparasitic nematodes can only be recovered from the soil and at least one stage of all endoparasites can be found in soil. It can be useful to collect plant roots in addition to soil when diagnosing crop damage because densities of endoparasitic nematodes are often greater inside roots than in soil during the growing season. Sending pictures of any foliar and root symptoms on the crop is also highly encouraged as it helps with diagnosis and recommendations. Symptoms to look for are described in the "Foliar Symptoms" and "Belowground Symptoms" sections below.
Soil samples for nematodes should be taken to about 12 inches deep. If plants or remnants of plants are still in the field, soil samples should be taken within a few inches of plant stems and should intersect plant roots if possible. Because nematode densities vary considerably across a field, about 20 soil cores, each of about 1-inch-diameter, should be taken from an area of 10 acres or less. Cores within a single area should be thoroughly mixed and a 1-pint portion of this mixture submitted for analysis. Multiple, separate samples can be taken to cover larger areas, making sure to properly label samples. If possible, avoid collecting samples when soil is excessively wet, such as when moisture is at field capacity after a heavy rain, or excessively dry, such as after a long drought. It is hard to collect and process samples in these conditions, and nematode densities in the upper soil layers may decline in these harsh conditions.
Soil samples should be stored in closed plastic bags to protect them from drying and should be kept cool, but not frozen, until shipment. Root samples should be dug and retained in the soil surrounding the roots. Retaining the soil around the roots protects them from decay by microbes. Root samples should be collected and stored in a similar manner to soil samples. If samples are intended to diagnose a current crop problem, it may be useful to collect separate samples from the diseased area and a healthy area for comparison.
While samples can be taken at any time of the year, nematode populations fluctuate throughout the year, so timing can be important. In most cases, nematode population densities peak around harvest while plant roots are still in the ground, so that is an ideal time to take routine or predictive samples. When diagnosing crop damage, take samples twice, first when damage is first observed and again around harvest. For questions about sampling for nematodes, contact your local extension agent, personnel of the UF/IFAS Nematode Assay Lab or the authors of this paper.
Foliar Symptoms
Symptoms of nematode infection in corn can be indistinct and difficult to detect, so routine sampling for nematodes, such as on a biannual basis, is especially important. Foliar symptoms of nematode infection may be similar to symptoms of nutrient deficiency or diseases and include stunted, chlorotic (yellowed) plants (Figure 5). In severe cases, nematode infestation—especially by sting or needle nematode—may kill young seedlings, reducing crop stand. These symptoms often occur in oval or irregular patches in the field, corresponding to areas of greater plant-parasitic nematode densities (Figure 6). These patches may surround the initial entry point for the nematodes or correspond to uneven environmental conditions, such as soil type. In fields infested with sting or needle nematodes, the spatial gradient from healthy to diseased plants may be clear and abrupt with apparently healthy plants next to severely stunted ones.

Credit: Zane Grabau, UF/IFAS

Credit: Zane Grabau, UF/IFAS
Belowground Symptoms
Stunted root systems and reduced yield are common, generalized symptoms of plant-parasitic nematode infection. Specific below-ground symptoms vary by nematode. Root-knot nematode infection typically causes galls—irregular swellings of the roots—but galling on corn is often small and indistinct, or even absent. These galls are caused by an increase in the size and number of cells triggered by root-knot nematode feeding. Galls contain one or more sedentary (immobile) adult female root-knot nematodes. These nematodes are contained in the roots and small—about 1 mm in diameter, so it is very difficult to view them in the field. However, with some practice, or the aid of a nematologist, it is possible to excise these pearly white females from the roots and look at them—a hand lens greatly aids viewing these small nematodes. Gall size will vary based on the root-knot species present and the severity of the infection; galls may cover much of the root surface if nematode densities are very great. Symptoms of root-knot nematode infection also vary by crop. As mentioned previously, galls are generally indistinct on corn, even when root-knot nematodes are thriving on the crop.
Nematode infection, particularly by lesion nematode, can cause dark lesions of dying (necrotic) tissue on corn roots (Figure 7). These lesions may join together to cover large portions of the root when infestations are severe. Fungal or bacterial pathogens may also infect dying root tissue. Sting, stubby-root, and needle nematodes often infect near the root tip. This may result in a severely stunted root system (Figure 8). Additionally, lateral roots may proliferate above the infection point, resulting in stunted roots with a hairy or bearded appearance (Figure 9). Nematode infection of lateral roots may make tips of these roots short, blunt, and swollen, giving roots a stubby appearance (Figure 10).

Credit: Tamra Jackson-Ziems, University of Nebraska-Lincoln. Used with permission

Credit: Zane Grabau, UF/IFAS

Credit: Zane Grabau, UF/IFAS

Credit: Zane Grabau, UF/IFAS
Management
Once plant-parasitic nematodes infest a field, it is not possible to eradicate them. Rather, the goals are to minimize crop damage by nematodes and keep nematode densities low, ideally at a level where no crop loss occurs. This is best achieved by employing a combination of management practices that are most effective and profitable for the given production situation based on nematode infestation level, other pest problems, available equipment, economics, and other considerations.
Exclusion
Exclusion is taking steps to stop or slow the spread of one or more plant-parasitic nematodes from infested to non-infested fields. Nematodes do not actively migrate from field to field, rather they are transported in infected soil, water, or plants. Wind and rain move these materials naturally, but they are also carried on field equipment. Besides avoiding intentionally moving soil or plant material, cleaning these materials from field equipment can slow nematode movement. This is particularly important when working both infested and non-infested areas. Nematodes can also be brought in on infected planting material. In general, this is not a large concern for row crops as corn nematodes do not infect seeds, but should be monitored if crops that are started as transplants are grown in the rotation.
Crop Rotation
Crop rotation can reduce nematode densities if a crop that the particular nematode cannot reproduce on (a non-host crop) or does not reproduce well on (poor host) is grown. In the absence of a host to reproduce on, the nematode population decreases as a portion of the nematodes die or are eaten.
Most plant-parasitic nematodes of corn have a wide host range or a host range that is not well-studied. For these reasons, it can be difficult to find suitable rotations for managing them. As an aid to selecting rotations for managing nematodes, Table 1 provides the host status of cash and cover crops for some most common and damaging nematodes in Florida corn production. If sting, stubby-root, or lesion nematodes are problematic on corn, grass species, such as sorghum, sugarcane, or grass forages, should be avoided as these nematodes thrive on grasses. Peanuts are a non-host of southern root-knot nematode (Meloidogyne incognita), so they are a good rotation crop if that nematode is causing problems. Weed management is an important supplement to crop rotation because plant-parasitic nematode population densities can be maintained or increased on weedy hosts, including volunteer corn, growing in a non-host crop.
Fallow and Cover Cropping
Offseason periods when a cash crop is not grown should also be considered as part of a crop rotation strategy. These periods are opportunities to either reduce or worsen a nematode problem. Fallowing fields in the offseason can help reduce nematode densities, but only if weeds, including volunteer soybeans, are controlled because many of them serve as hosts for nematodes. Growing an appropriate cover crop is usually best because erosion can be a major problem when fallowing fields, particularly on sandy soils.
If cover crops are grown, choosing a non-host or poor host for the nematodes present in the field is critical for nematode management (Table 1). Small grain cover crops, such as wheat, rye, and oats, are good hosts of sting nematode while susceptibility of these crops to root-knot nematodes tends to vary by cultivar.
Some cover crops—such as Brassicas (radish, mustards, etc.)—may have nematicidal properties, directly reducing densities of nematodes. However, most cover crops with nematicidal properties are not suitable or have not been tested as a winter cover crop in Florida. Susceptibility to nematodes should always be considered when selecting a cover crop, even one with nematicidal properties, because these cover crops can also be good hosts for some plant-parasitic nematodes, such as most Brassicas for root-knot nematodes. For further information on cover cropping for root-knot nematode management, see (https://edis.ifas.ufl.edu/in892).
Resistant Cultivars
Resistance is a trait that is selected for or bred in a cultivar; the given nematode can infect most cultivars of the crop to which the resistant cultivar belongs. Resistant cultivars sustain little or no damage from the target nematode, and growing resistant cultivars reduces field nematode population densities in a similar manner to non-host crops. No current commercial corn cultivars are resistant to any of the major plant-parasitic nematodes of corn. Some corn cultivars may yield better than others in the presence of nematodes, a characteristic called tolerance. Resistant cultivars of other crops, such as cotton resistant to southern root-knot nematode (Meloidogyne incognita) may be rotated with corn.
Nematicides and Other Commercial Products
Nematicides are chemical products intended to reduce nematode densities and protect crops from damage. Because field corn is a relatively low-value crop and nematicide application is a relatively expensive management technique, growers should consider whether nematicides are cost-effective at current prices before choosing to apply them. Determining the severity of nematode infestation in fields and considering spot-treating only those areas with heavy nematode infestation are ways to increase the likelihood of an economic return.
To protect workers, consumers, and the environment, nematicides must be used in a legal manner, as specified on the label, including on specified crops only. Two types of nematicides are registered for use on corn: (1) fumigants: broad-spectrum pesticides that move through soil as a gas, and (2) non-fumigants: granular or liquid products that affect only nematodes and, for some products, insects.
Three fumigants are labeled for corn: 1,3-Dichloropropene (1,3-D), metam sodium, and metam potassium (Table 2). In general, 1,3-D products are effective nematicides while metam sodium and metam potassium are inconsistent for nematode control at the lower rates that would be used on agronomic crops. Fumigant application is probably not cost-effective for field corn except in cases with severe infestation, because application is expensive, even at lower rates that are typically used for agronomic crops. Fumigant application also requires specialized equipment and detailed planning to conform with regulations.
Non-fumigants are easier and less expensive to apply than fumigants, although they are also generally less consistent and less effective for nematode control (Table 2). Most liquid and granular non-fumigant nematicides are available. Application at planting is preferred for these products because this timing optimizes nematode control while eliminating the cost of making a second tractor pass to apply products.
In addition to nematicides applied to soil or plants, some seed treatments (Avicta, Poncho/Votivo) are labeled for use against nematodes. These products are generally pre-coated on seeds and have not been tested extensively in Florida. Because seed treatments contain small amounts of product that is not widely distributed in the soil, they have a limited potential for nematode control. They may provide some early-season crop protection against nematodes, which may provide a return on investment. They are unlikely to provide adequate stand-alone control for nematode problems, particularly for heavy infestations.
Other Practices
Practices that promote plant health may help plants better tolerate nematode infection even if they do not reduce nematode populations. This includes practices such as maintaining soil fertility and quality, providing adequate water, and managing insects and diseases. Good knowledge of environmental and biological properties of soil at a site can also be a tool in nematode management. As described above, soil type and texture influence where nematode problems are likely to occur. Some soils, called suppressive soils, keep plant-parasitic nematode populations low without targeted management practices despite a susceptible crop and conducive soil. Practical and tested methods for developing these soils have not been developed, but they are likely caused in part by organisms that parasitize nematodes. Recognizing that a soil is suppressive can help cut costs by eliminating unnecessary management practices.
Selected References
Asmus, G., L. Ferraz, and B. Appezzato-da-Gloria. 2000. "Anatomical Changes in Corn (Zea mays L.) Roots Caused by Meloidogyne javanica." Nematropica 30:33–39.
Koenning, S., C. Overstreet, J. Noling, P. Donald, J. Becker, and B. Fortnum. 1999. "Survey of Crop Losses in Response to Phytoparasitic Nematodes in the United States for 1994." Journal of Nematology 31:587–618.
Oka, Y. 2010. "Mechanisms of Nematode Suppression by Organic Soil Amendments—A Review." Applied Soil Ecology 44:101–115.
Thomas, S., J. Schroeder, and L. Murray. 2005. "The Role of Weeds in Nematode Management." Weed Science 53:923–928.
Todd, T., and T. Oakley. 1996. "Seasonal Dynamics and Yield Relationships of Pratylenchus spp in Corn Roots." Journal of Nematology 28:676–681.
Wang, K., R. McSorley, R.N. Gallaher, and N. Kokalis-Burelle. 2008. "Cover Crops and Organic Mulches for Nematode, Weed and Plant Health Management." Nematology 10:231–242.
Windham, G.L. 1998. "Corn." p. 335–357. In K.R. Barker, G.A. Pederson and G.L. Windham (eds.) Plant and Nematode Interactions. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, WI.
Wyse-Pester, D., L. Wiles, and P. Westra. 2002. "The Potential for Mapping Nematode Distributions for Site-Specific Management." Journal of Nematology 34:80–87.