
Credit: J Newman, UF/IFAS/FMEL
Synopsis
Zika is a mosquito-transmitted virus that has spread broadly in tropical regions and caused epidemics, especially in the past 8–9 years. In its native range in West Africa and Uganda, Zika virus is maintained in forest cycles between infected treehole mosquitoes and arboreal primate hosts, with human infections regarded as incidental and medically inconsequential. A strain of the virus traced to outbreaks in French Polynesia emerged in northeastern Brazil in 2015 and provoked subsequent alarm because of increased incidence of microcephaly in babies born to Zika-infected mothers. Local transmission, mainly by the yellow fever mosquito Aedes aegypti, has now been documented in most tropical countries of the Americas and in the United States. Symptoms of mild infections include rash, fever, joint pain, and conjunctivitis, but molecular diagnoses of Zika infection are recommended for confirmation because of the similarity of clinical symptoms to those of other viral infections. Because no vaccine is available to prevent Zika infection, avoidance of areas where transmission has been detected and of mosquito bites, especially those of Aedes aegypti, are the best preventatives.
Zika Virus
Zika virus (ZIKV) disease is caused by an arthropod-borne (arbo) virus (Flaviviridae family genus Flavivirus, Spondweni serocomplex) consisting of three genetically distinct strains (lineages); one from Asia and two from Africa (Kuno et al. 1998, Faye et al. 2014). Zika virus is a positive sense single-stranded RNA molecule 10794 nucleotide bases long. Aedes species of mosquitoes become infected with ZIKV by feeding on the blood of an infectious vertebrate host (human or monkey) and may transmit the virus by bite to another human after an incubation period of several days. The virus was first isolated from Zika Forest of Uganda in 1947 from a sentinel rhesus monkey. The sylvatic (wild) transmission cycle occurs between canopy-inhabiting Aedes mosquito vectors and monkey hosts (Hayes 2009), whereas the urban cycle involves domestic Aedes mosquitoes (yellow fever and Asian tiger mosquitoes) and humans. The virus is spread usually by mosquito bite, but other modes of transmission are possible. Mothers infected with ZIKV may pass the virus to a fetus during pregnancy (Besnard et al. 2014, Schuler-Faccini et al. 2016). Additionally, Zika virus may be transmitted by blood transfusion and through sexual contact (Foy et al. 2011, Musso et al. 2014). Zika virus has been shown to be present in urine and saliva, but it is unclear whether it can be spread through these bodily fluids. Historically, ZIKV circulation was limited to tropical Africa and parts of Southeast Asia with only 14 known human infections prior to 2007 (Duffy et al. 2009, Hayes et al. 2009). Zika virus has experienced a geographic expansion over the last decade, spreading to islands of the Pacific region (since 2007) and the Americas (since 2015) (ECDC 2015), and causing epidemics. A large outbreak of Zika fever caused by the Asian lineage of ZIKV occurred in 2013–2014 in French Polynesia (Lanciotti et al. 2007, Musso 2015), and then spread to other Pacific Islands (Musso et al. 2015). Subsequently ZIKV spread to Brazil, perhaps associated with international travel for athletic competitions in Brazil (Zanluca et al. 2015). The outbreak in Brazil, and other countries and territories in the Americas, is attributable to the Asian lineage of ZIKV (Zanluca et al. 2015).
Mosquito Vectors of Zika Virus
Forest-dwelling species of the genus Aedes, especially Aedes africanus in Uganda (Haddow et al. 1964), and Aedes furcifer, Aedes luteocephalus, and Aedes africanus in Senegal (Diallo et al. 2014), maintain the African Zika cycle between mosquitoes and arboreal primates. Males of Aedes furcifer caught infected in the wild provide evidence that Zika can be transmitted from an infected female vector to her offspring through the egg stage (Diallo et al. 2014).
Serological investigations showed that human infections with Zika can be common. For example, 40% of an urban population in Nigeria had antibodies to the virus (Fagbami 1979). During spillovers from the forest cycle into domestic or cleared habitats, other Aedes species have been found infected with Zika, such as Aedes metallicus and Aedes vittatus in SE Senegal (Diallo et al. 2014). The epidemiological importance of Zika isolations in Senegal from mosquitoes of other genera, notably species of Mansonia, Culex, and Anopheles, remains unclear (Diallo et al. 2014).
In the invasive range of the virus outside of Africa, the most likely vector of Zika is Aedes aegypti, first recognized as infected in nature in Malaysia (Marchette et al.1969). The preference of domestic Aedes aegypti for feeding on human hosts in tropical cities overrides its inefficiency in developing the ZIKV (Diagne et al. 2015). Aedes albopictus has been implicated as a Zika vector in Gabon (Grard et al. 2014) and has expanded its invasive range worldwide. This finding assumes greater importance because both the mosquito and Zika virus are extending their range and thus it is possible that human-feeding populations of this vector species may co-occur with the emergent virus. In Micronesia, the endemic Aedes hensilli was the probable vector of Zika during the 2007 epidemic of the disease on Yap Island (Duffy et al. 2009).
Which mosquito species native to the Americas might become vectors of Zika remains to be determined by future research. Concern is justified that Zika could become endemic in tropical New World forests, in transmission cycles between arboreal treehole mosquitoes, such as species of Haemagogus and Sabethes, and Neotropical non-human primates, just as occurred in South and Central America for yellow fever virus centuries earlier after its introduction and spread from Africa (Barrett and Higgs 2007). The relative importance of different vector species in the current epidemics in the Americas is unknown, but Aedes aegypti and Aedes albopictus are the most probable transmitters. If confirmed, their capacity to transmit the virus would increase concern in other areas where these species are present.
Zika Virus Disease
Symptoms: The symptoms are usually characterized as sudden onset of fever with rash, joint pain, and conjunctivitis (pink eye), and may include muscle aches and headache. Until recently, illnesses from ZIKV have been classified as clinically mild with symptoms lasting several days to a week, with many infected individuals being asymptomatic (80%, CDC 2016). Hospitalizations and fatalities were uncommon. However, the 2013 ZIKV outbreak in French Polynesia implicated ZIKV in neurological complications resulting in Guillain-Barré syndrome, and the recent ZIKV outbreak in Brazil is associated with an increase in the number of babies born with microcephaly (ECDC 2015; PAHO/WHO 2015, 2016).
Diagnoses: Zika virus infections are sometimes hard to diagnose because the disease has symptoms similar to other arboviral diseases, and few laboratories have molecular tests for ZIKV. A combination of assessments is used to determine presence of ZIKV-associated disease. Outside areas of active transmission, preliminary diagnosis includes obtaining travel history. The laboratory assessment of infection status involves detection of ZIKV nucleic acid in serum (by reverse transcriptase-polymerase chain reaction) during the initial onset of symptoms. At the end of the first week with symptoms, IgM antibody against ZIKV (detected by ELISA) and ZIKV plaque reduction neutralization test 50 (a test used to quantify the titer of the neutralizing antibody for a virus) can be used to verify exposure to ZIKV (Duffy et al. 2009; CDC 2016). Zika virus can be detected in human blood as early as the day symptoms appear, and viral RNA detected as late as 11 days after symptoms begin (Lanciotti et al. 2008).
Treatment: Because there is no vaccine or medication available to treat ZIKV infections, treatment involves supportive care and includes rest, drinking plenty of fluids, and medication to relieve fever and pain, such as acetaminophen. Patients should not take aspirin or other non-steroidal anti-inflammatory drugs until dengue has been ruled out because these drugs may aggravate bleeding associated with some forms of dengue (CDC 2016).
Zika and Microcephaly: There may be an association between the presence of Zika virus and congenital microcephaly in newborn babies. Microcephaly describes a rare neurological condition in which the head of the infant is smaller than the heads of infants of the same age and sex due to abnormal development of the brain in the womb. In October 2015, the Brazilian Ministry of Health reported an increase in the number of babies born with microcephaly coincident with an outbreak of ZIKV in May (ECDC 2015; PAHO/WHO 2015; 2016). Evidence of ZIKV was found in the amniotic fluid drawn from two pregnant women whose fetuses were diagnosed in utero as having microcephaly and later identified in the brain and in placenta (PAHO/WHO 2015). At time of publication no definitive link between ZIKV infection and occurrence of microcephaly has been made (Fauci and Morens 2016) ; nevertheless, during the current (2015–2016) Zika outbreak, several health agencies including the Centers for Disease Control and Prevention have advised pregnant women not to travel to locations where there is active transmission of the virus.
Zika virus and Guillain-Barré syndrome: Guillain-Barré syndrome (GBS) occurs when a person's immune system attacks their nerve cells, causing muscle weakness and sometimes paralysis. There are approximately 3,000–6,000 cases annually in the United States.
The cause of GBS is not well understood, but many GBS cases occur shortly after an infection with a virus or bacteria. GBS has been observed after infection with many different pathogens, including mosquito-borne viruses such as dengue or chikungunya (Lebrun et al. 2009; Oehler et al. 2015). GBS in Polynesia was associated with previous ZIKV infection where the incidence of GBS increased during the Zika epidemic (Oehler et al. 2014); many GBS cases had symptoms consistent with ZIKV infection (PAHO 2016). Recent GBS cases from Brazil followed ZIKV infection, and El Salvador has reported an unusual increase in GBS since December 2015 (PAHO 2016). However, the underlying cause of GBS, and how Zika virus or other arboviral infections are involved, remain unknown.
History of Zika Virus
The virus was named after Zika Forest, where it was first discovered by scientists working at the nearby Yellow Fever Research Institute in Entebbe, Uganda. Initial isolates came not only from the sentinel monkey, caged on a tree platform in this forest, but also from Aedes africanus mosquitoes captured while feeding on humans (Dick et al. 1952). These isolations, and the absence of evidence of this virus among small mammals captured from the forest floor, led to the hypothesis that Zika was maintained in nature between arboreal primates and tropical mosquito species that typically bloodfeed in the forest canopy, not unlike the sylvan cycle of the related yellow fever virus (Haddow et al. 1964). Scarce documented infections of humans were regarded as dead-ends that did not contribute to the natural cycle of Zika (Simpson 1964).
Zika was later identified from multiple countries in West and Central Africa, where it was also believed to persist in forest cycles between arboreal mosquito vectors and simian hosts. Molecular analyses of ZIKV lineages suggested two introductions from Uganda into West Africa, the first beginning around 1935 and the second around 1940 (Faye et al. 2014; Figure 1).
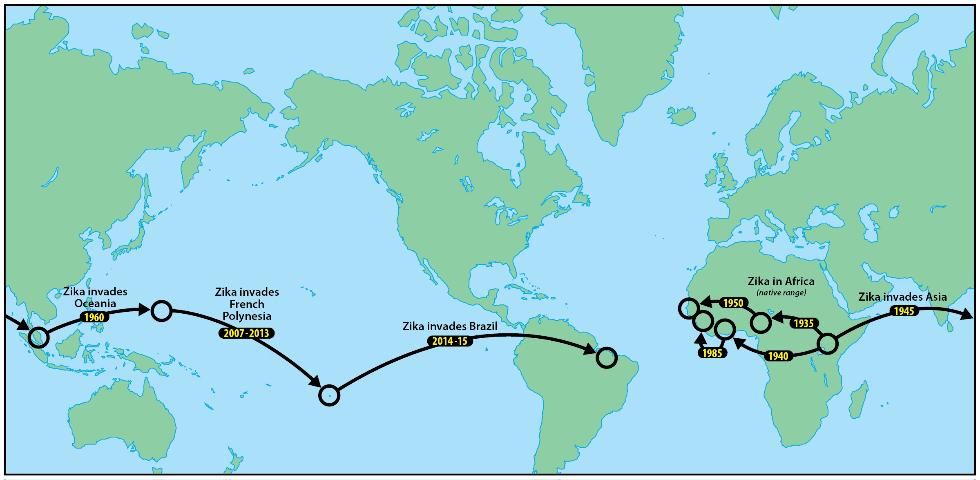
Credit: Jim Newman, UF/IFAS/FMEL
Evidence of Zika in Pakistan, Malaysia, and Indonesia indicated that Zika became established in Asia, perhaps around 1960 (Faye et al. 2014). This Asian lineage was believed to be the source of an introduction of Zika to Yap Island, Micronesia, which resulted in 2007 in the first epidemic attributable to this virus (Duffy et al. 2009). Although the 49 clinical cases on Yap had relatively mild symptoms (rash, fever, arthritis, conjunctivitis), household surveys validated that more than 73% of the inhabitants of the island had been infected and that this pathogen could easily be transported by infective travelers to other islands in Oceania, or even to the Americas (Hayes 2009).
The emergence of Zika outside of Africa coincided with an apparent change in predominant transmission cycle, from humans being incidental hosts outside the sylvan cycle between monkeys and feral mosquitoes, to humans being the primary hosts, where the virus is primarily vectored by domestic or peridomestic mosquitoes such as invasive Aedes aegypti, and Aedes albopictus.
A larger outbreak of Zika in French Polynesia during 2013, also derived from the Asian lineage, followed the epidemic in Yap (Cao-Lormeau et al. 2014). An epidemic in northeastern Brazil in early 2015 (Campos et al. 2015) is suspected to have begun with the introduction of Zika by a traveler from French Polynesia (Musso 2015). More than twenty countries in the tropical Americas, as well as the United States and the Commonwealth of Puerto Rico and the US Virgin Islands, currently recognize active ZIKV transmission (CDC 2016).
Zika in Florida
At the time of this report, local transmission of ZIKV in the United States and its territories has been reported in Texas (by sexual transmission), Commonwealth of Puerto Rico, the US Virgin Islands, and American Samoa. There have been multiple imported Zika fever cases in Florida in 2016 associated with travel to Colombia, El Salvador, Haiti, and Venezuela; as of February 5, 2016, four from Miami-Dade, two from Hillsborough, two from Lee, and one from Santa Rosa Counties (Florida Department of Health 2016). The geographic expansion of ZIKV, especially in the Americas, poses a risk for local transmission of ZIKV in Florida attributable to
1) an increase in travel-associated imported cases of ZIKV in Florida, a tourism state;
2) large populations of susceptible humans especially in urban areas;
3) environmental conditions conducive for mosquito development and virus replication, especially during the rainy season; and
4) abundant vectors Aedes aegypti and Aedes albopictus associated with human populations throughout much of the state.
There were 14 imported cases of Zika fever in the United States in 2007–2014, as documented by the CDC. The spread of ZIKV to the Americas has been associated with an increase in the number of imported cases to the United States. Imported cases increase the risk for the establishment of local transmission because the virus is present in the blood of infected individuals for several days before recovery, potentially exposing the person to biting mosquitoes which could become infected and spread the virus to other people.
Prevention of Zika Infection
No vaccines for protection against Zika are currently available, so protecting against mosquito bites is the primary method of prevention. Given the possibility that the virus may be sexually transmitted, couples should take precautions against direct transmission of the virus from their partners (see https://www.cdc.gov/media/releases/2016/s0205-zika-interim-guidelines.html). While several candidate vaccines are in development, FDA-approved vaccines are not likely to be available for several years. Protection against mosquito bites involves personal protection and mosquito population reduction. Personal protection against mosquito bites may be achieved through the use of mosquito repellents, protective clothing, and avoiding areas where mosquitoes are abundant. The CDC provides a list of mosquito repellents with Environmental Protection Agency-registered active ingredients, such as DEET, picaridin, and oil of lemon eucalyptus that provide long-lasting protection (https://www.cdc.gov/westnile/prevention/index.html). The mosquito species that carry ZIKV outside of Africa thrive in urban and suburban areas. Their larvae develop in water that collects in natural and manmade containers such as birdbaths, vases, animal water dishes, flower pots, discarded cans, old paint buckets, or other neglected/discarded water-holding objects (Rey and Connelly 2013). Regularly removing and/or emptying such water-holding containers (Connelly et al. 2014; https://edis.ifas.ufl.edu/publication/in1045) are effective ways of reducing the number of mosquitoes that might transmit ZIKV. Suspected Zika infections are mandatory reportable diseases, as classified by the CDC. This means that any health professional in the United States who suspects that a patient is infected with ZIKV is compelled by law to report their suspicion to the proper health authorities (state health department and/or CDC). This information is then sent to appropriate agencies, which typically include local mosquito control districts. Mosquito control districts can act on this information to suppress mosquitoes in areas where suspected ZIKV cases occurred. Mosquito control districts in Florida may provide additional information on the mosquitoes that transmit ZIKV that will help to determine local risks of ZIKV infection. The Florida Medical Entomology Laboratory, a Research and Education Center of the University of Florida that specializes in mosquito-transmitted pathogens, provides a list of the 61 Mosquito Control Programs in Florida, with links to websites for many of the programs https://fmel.ifas.ufl.edu/florida-mosquito-control/florida-mosquito-control-table-by-county/.
References
Ahmed, A. 2016. "One country's advice on Zika virus: don't have babies." New York Times 25 January 2016.
Barrett, A. D. H., and S. Higgs. 2007. "Yellow fever: a disease that has yet to be conquered." Annual Review of Entomology 52: 209–229.
Besnard, M., S. Lastère, A. Teissier, V. M. Cao-Lormeau, and D. Musso. 2014. "Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014." Euro. Surveill. 19(13): ppii: 20751.
Campos, G. S., A. C. Bandeira, and S. I. Sardi. 2015. "Zika virus outbreak, Bahia, Brazil." Emerging Infectious Diseases 21: 1885–1886.
Cao-Lormeau, V-M., C. Roche, A. Tessier, E. Robin, A-L. Berry, H-P. Mallett, A. A. Sall, and D. Musso. 2014. "Zika virus, French Polynesia, South Pacific, 2013." Emerging Infectious Diseases 20: 1085–1086.
Carod-Artal, F., O. Wichmann, J. Farrar, and G. Joaquim. 2013. "Neurological complications of dengue virus infection." Lancet Neurol. 12: 906–916
Centers for Disease Control and Prevention (CDC). 2016. Zika Virus. National Center for Emerging and Zoonotic Infectious Diseases (NCEZID). https://www.cdc.gov/zika/ (accessed 4 April 2022)
Connelly, C. R., E. Bolles, D. Culbert, J. DeValerio, M. Donahoe, K. Gabel, R. Jordi, J. McLaughlin, A. S. Neal, S. Scalera, E. Toro, and J. Walter. 2014. Integrated Pest Management for Mosquito Reduction around Homes and Neighborhoods. https://edis.ifas.ufl.edu/publication/in1045 (accessed 4 April 2022)
Diagne, C. T., D. Diallo, O. Faye, Y. Ba, O. Faye, A. Gaye, I. Dia, O. Faye, S. C. Weaver, A. A. Sall, and M. Diallo. 2015. "Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus." BMC Infectious Diseases 15: 492.
Diallo, D., A. A. Sall, C. T. Diagne, O. Faye, O. Faye, Y. Ba, K. A. Hanley, M. Buenemann, S. C. Weaver, and M. Diallo. 2014. "Zika virus emergence in mosquitoes in southeastern Senegal." PLoS One 9(10): e109442.
Dick, G. W. A., S. F. Kitchen, and A. J. Haddow. 1952. "Zika virus." Transactions of the Royal Society of Tropical Medicine and Hygiene. 46: 509–534.
Duffy, M. R., T-H. Chen, W. T. Hancock, A. M. Poers, J. L. Kool, R. S. Lanciotti, M. Pretrick, M. Marfel, S. Holzbauer, C. Dubiay, L. Guillaumot, A. Griggs, M. Bel, A. J. Lambert, J. Laven, O. Kosoy, A. Panella, B. J. Biggerstaff, M. Fischer, and E. B. Hayes. 2009. "Zika virus outbreak on Yap Island, Federated States of Micronesia." New England Journal of Medicine 360: 2536–2543.
[ECDC] European Centre for Disease Prevention and Control. 2015. Rapid risk assessment: Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome. 10 December 2015. Stockholm: ECDC, 2015.
Fagbami, A. H. 1979. "Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State." The Journal of Hygiene 83: 213–219.
Fauci, A. S., and D. M. Morens. 2016. "Zika Virus in the Americas — Yet Another Arbovirus Threat." The New England Journal of Medicine. DOI: 10.1056/NEJMp1600297
Faye, O., C. C. M. Freire, A. Lamarino, O. Faye, J. V. C. de Oliveira, M. Diallo, P. M. A. Zanotto, and A. A. Sall. 2014. "Molecular evolution of Zika virus during its emergence in the 20th century." PLoS Neglected Tropical Disease 8(1): e2636.
Florida Department of Health. 2016. https://www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/_documents/week4arbovirusreport-1-30-16.pdf (site visited April 14, 2022).
Foy, B. D., K. C. Kobylinski, J. L. Chilson Foy, B. J. Blitvich, A. Travassos da Rosa, et al. 2011. "Probable non-vector-borne transmission of Zika virus, Colorado, USA." Emerg. Infect. Dis. 17(5): 880–882.
Grard, G., M. Caron, I. M. Mombo, D. Nkoghe, S. M. Ondo, D. Jiolee, D. Fontenille, C. Paupy, and E. M. Leroy. 2014. "Zika virus in Gabon (Central Africa)-2007: a new threat from Aedes albopictus?" PLoS Neglected Tropical Diseases 8(2): e2681.
Haddow, A. J., M. C. Williams, J. P. Woodall, D. I. H. Simpson, and L. K. H. Goma. 1964. "Twelve isolates of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest." Bulletin of the World Health Organization. 31: 57–69.
Hayes, E. B.. 2009. "Zika virus outside Africa." Emerging Infectious Diseases 15: 1347–1350.
Kuno, G., G. J. Chang, K. R. Tsuchiya, N. Karabatsos, and C. B. Cropp. 1998. "Phylogeny of the genus Flavivirus." J. Virol. 72(1): 73–83.
Lanciotti, R. S., O. L. Kosoy, J. J. Laven, J. O. Velez, A. J. Lambert, A. J. Johnson, S. M. Stanfield, and M. R. Duffy. 2008. "Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007." Emerg. Infect. Dis. 14(8): 1232–1239.
Lebrun, G., K. Chadda, A. Reboux, O. Martinet, and B. Gaüzère. 2009. "Guillain-Barré syndrome after Chikungunya infection." EID 15: 495–496.
Marchette, N. J., R. Garcia, and A. Rudnick. 1969. "Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia." American Journal of Tropical Medicine and Hygiene 18: 411–415.
Mayo Clinic. 2016. "Disease and Conditions: Microcephaly." https://www.mayoclinic.org/diseases-conditions/microcephaly/symptoms-causes/syc-20375051
Musso, D. 2015. "Zika virus transmission from French Polynesia to Brazil." Emerging Infectious Diseases 21: 1887.
Musso, D., V. M. Cao-Lormeau, and D. Gubler. 2015. "Zika virus: following the path of dengue and chikungunya?" Lancet 386(9990):243–44.
Musso, D., T. Nham, E. Robin, C. Roche, D. Bierlaire, K. Zisou, A. Shan Yan, V. M. Cao-Lormeau, and J. Broult. 2014. "Potential for Zika virus transmission through blood transmission demonstrated during an outbreak in French Polynesia, November 2013 to February 2014." Euro Surveill. 19(14): ppii: 20761.
National Institutes of Health. "NIH GBS fact sheet." https://www.ninds.nih.gov/health-information/disorders/guillain-barre-syndrome (accessed 1 February 2023)
Oehler, E., L. Watrin, P. Larre, I. Leparc-Goffart, S. Lastère, F. Valour, L. Baudouin, H. P. Mallet, D. Musso, and F. Ghawche. 2014. "Zika virus infection complicated by Guillain-Barré syndrome – case report, French Polynesia, December 2013." Euro Surveill. 19(9):pii=20720. Article DOI: https://doi.org/10.2807/1560-7917.ES2014.19.9.20720
Oehler, E., E. Fournier, I. Leparc-Goffart, P. Larre, S. Cubizolle, C. Sookhareea, S. Lastère, and F. Ghawche. 2015. "Increase in cases of Guillain-Barré syndrome during a Chikungunya outbreak, French Polynesia, 2014 to 2015." Euro Surveill. 20(48):pii=30079. DOI: https://doi.org/10.2807/1560-7917.ES.2015.20.48.30079 (accessed 5 February 2016)
Pan American Health Organization, World Health Organization. Regional Office for the Americas. 2015. "Epidemiological Alert: Increase of microcephaly in the northeast of Brazil." https://iris.paho.org/handle/10665.2/50666
PAHO/WHO. 2016. Epidemiological update: neurological syndrome, congenital anomalies and Zika virus infection. https://iris.paho.org/handle/10665.2/50659.
Rey, J. R., and C. R. Connelly. 2013. Florida Container Mosquitoes. https://edis.ifas.ufl.edu/in851 (accessed 5 February 2016)
Schuler-Faccini, L., E. M. Ribeiro, I. M. L. Feitosa, D. D. G. Horovitz, D. P. Cavalcanti, et al. 2016. "Possible association between Zika virus infection and microcephaly – Brazil, 2015." MMWR Morb. Mortal. Wkly. Rep. 65(3): 59–62.
Simpson, D. I. H. 1964. "Zika virus infection in man." Tropical Medicine and Hygiene 58: 335–338.
Zanluca, C., V. C. A. de Melo, A. L. P. Mosimann, G. I. V. dos Santos, C. N. D. dos Santos, and K. Luz. 2015. "First report of autochthonous transmission of Zika virus in Brazil." Mem Inst Oswaldo Cruz. 110(4): 569–572.