The purpose of this publication is to inform, educate, and recommend applicable ways to reduce and/or prevent the spread of blueberry gall midges on blueberry farms in Florida. This is done by providing information about the pest’s biology, behavior, and damage potential. This publication shares information on the preventative course of action to take prior to and during midge infestation, as well as recommendations for effective integrated pest management (IPM) strategies to manage this pest. This publication is intended for blueberry growers who have farms affected by the blueberry gall midge. Conventional growers who have southern highbush blueberries will directly benefit by gaining knowledge on effective insecticides with lower detrimental effects on important natural enemies.
Introduction
Blueberry gall midges (BGM), Dasineura oxycoccana (Johnson) (Diptera: Cecidomyiidae) are important pests of the Vaccinium species. The injury that the larvae induce manifests as crumpled and withered buds (Figure 1), leading to reduced plant vigor, increased susceptibility to secondary infections, and reduced yields by up to 80% of buds (Dernisky et al. 2005; Lyrene and Payne 1992). In Florida, populations of BGM have been recorded on blueberry farms throughout the north-central and central regions. Infestations are also more commonly observed in nurseries than on farms (Hahn and Isaacs 2012).

Credit: M. Lopez, UF/IFAS
Blueberry gall midges are not particularly uniform in distribution throughout a field. It is a common misconception that BGM swarm or form clouds in the air on hot days, but the swarms people observe are generally other, non-pest flies. It is recommended to begin monitoring for BGM as early as mid to late November.
Preventive control measures include maintaining blueberry beds by regularly adding fresh mulch or employing polyethylene plastic or fabric mulch; selecting cultivars that exhibit greater resistance to BGM; and ensuring proper timing of reduced-risk insecticide applications prior to the onset of floral bud break, followed by another application ten days later to safeguard natural enemies within the system. Chemical control measures can also be employed when two or more adult BGM are seen in a trap (Liburd and Phillips 2019).
Background
Having a comprehensive understanding of the phenological characteristics of the BGM is essential for mitigating potential yield losses in the subsequent season. Adults live for two to three days, and once mated, females lay eggs in the flower and leaf buds. While females typically choose buds that are in their second to third developmental stage (Sampson et al. 2002; Lyrene and Payne 1995) (Figure 3), they have been observed laying eggs as early as the first developmental stage (Figure 2). One bud can contain eggs from multiple females, and up to 33 larvae have been observed emerging from a single bud (findings from M. Lopez PhD research). The larvae take around 10 days to develop, after which they emerge from the buds and undergo pupation in the soil (Bosio et al. 1998). Under ideal temperatures, BGM can complete a generation within a short timeframe of two to three weeks (Bosio et al. 1998).

Credit: M. Lopez, UF/IFAS
The damage inflicted by BGM on blueberry and cranberry flower buds has been mistakenly attributed to freeze damage or insufficient chilling (Lyrene and Payne 1995). In recent years, growers faced “false winters” beginning in November. During false winters, there is a cold period when temperatures fall between 20°C–25°C (68°F–77 °F) for one to two days, then quickly rise again. Blueberry gall midges break out of their summer and fall diapause state after one of these sudden chilling events. Because males emerge before females, finding mostly males in a planting is a tell-tale sign that the midges have just begun to exit diapause; more females will likely be seen after two weeks (Liburd and Phillips 2019). If there are subsequent warm temperatures, the first generation of eggs will hatch, and larvae will begin feeding inside the buds, which damages flowers and leaves (Dernisky et al. 2005). If the development is allowed to proceed, BGM that emerged due to false winters may also contribute to higher spring populations, causing more significant yield losses in the upcoming season. In Mississippi, Sampson et al. (2000) recorded up to 11 generations annually. However, no such studies have been conducted in Florida, and the accelerated development resulting from warmer winter months introduces the possibility of additional undocumented generations.
Monitoring and Management Practices
Monitoring for BGM and implementing chemical controls should commence following a cold spell or as soon as the first generation is observed. Effective monitoring techniques, such as destructive sampling and the utilization of multiple traps, can be employed to detect the presence of BGM.
Destructive Sampling
It is recommended to collect flower buds when they are in their second to third developmental stages. During this period, flower buds swell, and bud scales begin to separate—also known as “bud break” (Figure 3). (Refer to EDIS Publication, “Reproductive Growth and Development of Blueberry” (Phillips et al. 2020) for more information). In addition, collecting buds in the late afternoon or early evening hours gives the collector a better representation of what is in the system. During this time of the day, most females have laid their eggs, and larvae are well hidden inside the buds, protected from the hot day-time conditions (findings from M. Lopez PhD research). Most larvae exit the buds and pupate in the soil from early to mid-morning (6 a.m.–9 a.m.) (Lopez n.d.)), when morning dew protects the larvae from drying out on their way down from flower and leaf buds (Figure 4). Buds can be placed in a zip lock bag and kept at room temperature. After two to four days, larvae will begin to emerge. While most larvae will emerge in the first few days, it can take up to two weeks for all the larvae to emerge from the buds.
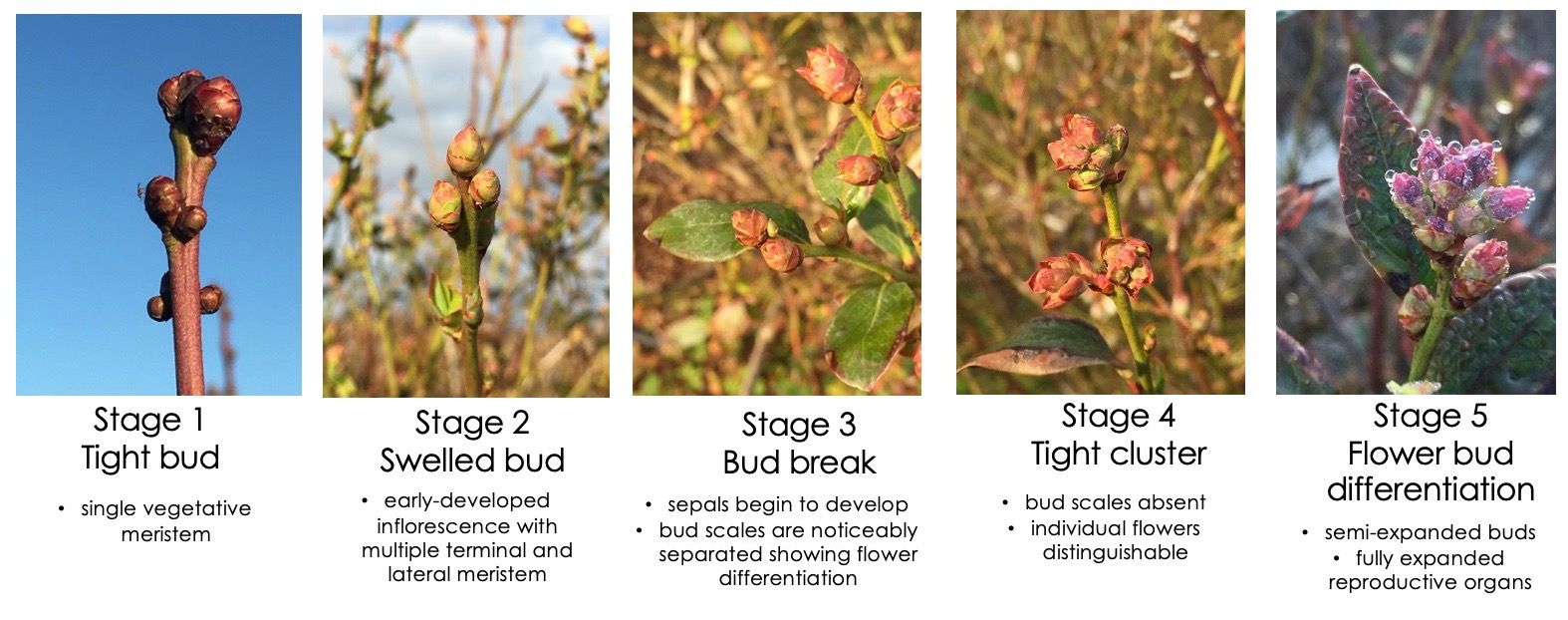
Credit: M. Lopez, UF/IFAS

Credit: M. Lopez, UF/IFAS
Trapping
Clear, sticky, adult emergence traps are a cost-effective and informative monitoring strategy for BGM. Use these traps to detect mature BGM before they lay eggs in buds (Hahn and Isaacs 2012; Roubos and Liburd 2010). Construct a trap by cutting a 12”x 12” clear, sturdy plastic sheet into four smaller sheets. Punch a hole at the top of the sheet to hang it on the bush, and spray the trap with a sticky product, such as Tanglefoot® Tangle Trap Sticky Coating (Oro Valley, AZ). Hang sticky sheet traps in the middle to lower canopy of the blueberry bushes throughout the field (Figure 5). These traps remain sticky for one to two weeks in the field, depending on weather conditions. Once collected, cover the traps with plastic wrap such as cling wrap. This allows for easier handling and identification of insects with a hand lens or under a stereomicroscope. Wrapping sticky traps with cling wraps also helps to preserve the state of insect bodies for long-term storage.

Credit: M. Lopez, UF/IFAS
Alternatively, bucket emergence traps are another method to monitor for the presence of BGM (Roubos and Liburd 2010) (Figure 6). These traps can effectively detect low populations of BGM (Rhodes et al. 2014) and can accurately tell the grower if a new generation of larvae or adults have emerged on the same day they are deployed. Set up the traps early in the morning and check them after a few hours. The orange larvae will be clearly visible stuck to the top of the plexiglass. By noon, most of the larvae from the bush above will have fallen onto the top of the plexiglass of the bucket trap. The adults emerge from the soil below the bucket traps throughout the day, but most will be in higher numbers between mid-morning to mid-afternoon hours (9 a.m.–3 p.m.). Adults stick to the underside of the plexiglass, and growers can observe their distinctive orange bodies with a hand lens (Figure 7A–E, Diagnostic Characteristics). These traps are a little more costly and cumbersome to handle than sticky traps. However, bucket emergence traps have the advantage of directly trapping adults and catching larvae that have recently dropped to the ground and eclosed (i.e., departed from their pupal shell).
Bucket emergence traps are constructed from the bottom half of 5-gallon buckets. Cut a cut out of the bottom of the bucket. Place a plexiglass hexagon, slightly larger than the circle, over the hole, secured with pins, and sprayed with Tangle Trap on both sides. Place these directly below the dense foliage of blueberry bushes, about 16–20 inches (40–50 cm) from the trunk of the bush (Rhodes et al. 2014; Roubos and Liburd 2010) (Figure 6).

Credit: M. Lopez, UF/IFAS
When placing traps in the field, growers should consider “hot spots” where midges can develop, indicated by depressions in the ground. Growers observed the highest infestations in these lower-lying areas, possibly due to trapped air pockets warmer than the surrounding environment. Growers can have between two to four clear sticky traps and one to three bucket emergence traps per acre. It is ideal to check the traps once every few days or once a week. Chemical control is recommended when two or more BGM are caught on both traps (Liburd and Phillips 2019).
Diagnostic Characteristics
To confirm the presence of blueberry gall midge, a microscope is required as their minuscule morphological characteristics are hard to see under a hand lens (Figure 7A–E). Both sexes are around 2–3 mm long, have orange abdomens, and bear black stripes across their tergites (top side of their abdomen) (Figure 7A). Both sexes also have a distinctive R5 vein curved upward on their forewings (Figure 7B), which is different from the R5 vein on forewings of Prodiplosis vaccinii (Felt) (Figure 7C), another pest of blueberries. Males are easily distinguishable from females by a forceps-like structure on their genitalia at the end of their abdomen and bead-like antennae with sparse clusters of sensory hairs, called sensilla, on each antennal segment of the flagellum (Figure 7D). The longest part of the antennae, the flagellum, is more visibly subdivided in its parts in males than in females.
Females are slightly larger, have less visible sensilla, have more compact moniliform antennae, and have a noticeably swollen orange abdomen (Figure 7A, E). Though the ovipositor is normally not visible, females can extend it to almost the length of their body to show receptiveness before mating (personal observation).
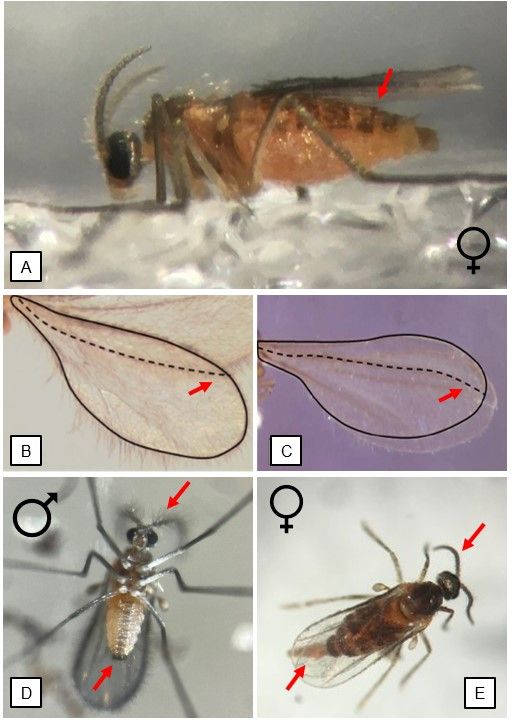
Credit: (7A, D–E) M. Lopez; (7B–C) C. Roubos, UF/IFAS
Management Practices
Management practices for BGM include the use of mulches, potentially resistant cultivars, and natural enemies. Use reduced-risk and conventional insecticides as a last resort when all other strategies have proven ineffective.
Mulches
Populations of BGM thrive in older, more decomposed mulch. This is because when the larvae exit the bud, they need to reach the soil level as quickly as possible to prevent their bodies from desiccating. Irrigation in the morning can potentially expedite their journey down the bush and in between the soil particles. It is advisable to keep mulches below the 75% decomposed stage (Figure 8).
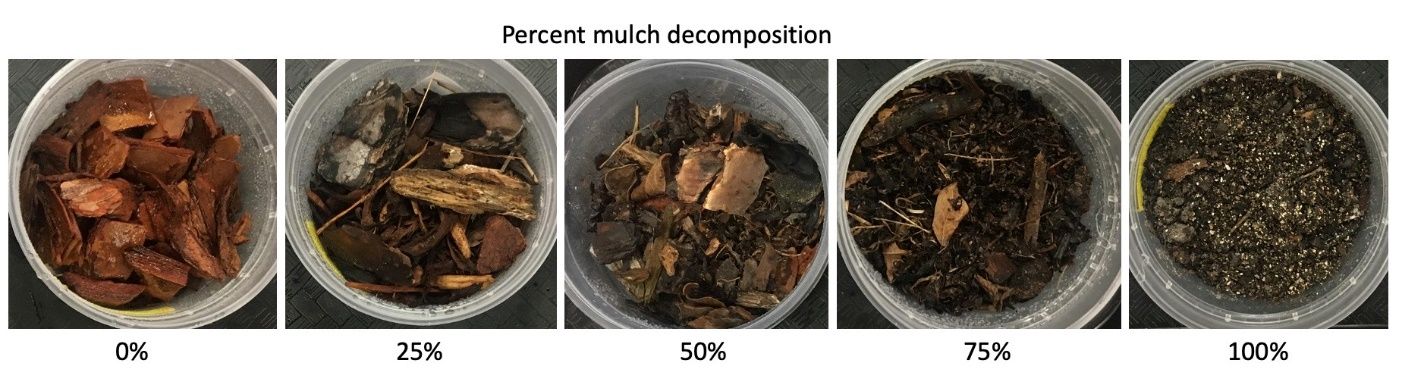
Credit: M. Lopez, UF/IFAS
Conventional growers have the option to use black or white polyethylene plastic mulch. Because BGM need a place to burrow in the soil, it is almost impossible for the larvae to reach the soil when plastic mulches are used over the raised beds. Only when the bushes grow past the plastic mulched area, or if larvae exit the buds during rainfall or irrigation, can larvae reach the soil in the alleyways and continue their life cycle through pupation and emergence.
Potentially Resistant Cultivars
Since the 1970s, the UF blueberry breeding program has successfully developed commercial varieties to meet Florida’s growing conditions. To maintain high-quality competitive yields, growers are looking into more resilient cultivars that are resistant to the injury and damage caused by the BGM.
Six southern highbush cultivars were screened for resistance against this midge in north-central Florida, including ‘Farthing’, ‘Patrecia’, ‘Magnus’, ‘Sentinel’, ‘Optimus’, and ‘Colossus’. Field screening and laboratory host preferences assays were conducted from November to March from 2021–2022 between the bud swell and late bud break stages of development. Research by M. Lopez (n.d.) indicates that ‘Sentinel’ and ‘Optimus’ may have high levels of resistance, while ‘Magnus’ and ‘Colossus’ have moderate levels. ‘Farthing’ and ‘Patrecia’ appear to have the lowest levels of resistance (Table 1). The poor resistance of these two latter cultivars to BGM needs to be further studied.
‘Farthing’ is an older cultivar (released in 2007) and has been planted alongside ‘Patrecia’ for a longer time than the other cultivars. Over many generations, the midges in these plantings may have evolved to synchronize their larval-stage growth time (i.e., degree day requirements) with the cultivars’ dormancy time. Thus, after the plants break out of dormancy, flowers from ‘Farthing’ and ‘Patrecia’ are at the critical stage for adult females to successfully oviposit in them. More research is needed to test this hypothesis.
Regarding other cultivars, ‘Emerald’ and ‘Jewel’ suffered major crop losses on a central Florida farm due to severe BGM infestations, while infestation levels were low to moderate on the same cultivars on other farms in the north-central region. Temperature and relative humidity, the length of planting establishment time, the cultivar planted, and cultural control strategies all play a role in how severe a BGM infestation will be. We predict that plantings established for more than five years with little mulch turnover will host greater numbers of BGM. With warmer winters, more generations of this pest can exist in one season and lead to reproductive isolation, where the pest can potentially develop a close relationship with a specific cultivar.
Table 1. Rating scale given to eight southern highbush blueberry cultivars.
Natural Enemies
In organic plantings, spiders have been observed constructing webs around the vulnerable blueberry buds and early floral development stages (Figure 9). Spiders may play a significant role in minimizing BGM numbers, but further research is warranted.

Credit: M. Lopez, UF/IFAS
Parasitoids are the most well-known natural enemies of BGM. These tiny micro-wasps are between 1.2–4 mm long and hard to distinguish from other small insects with the naked eye. They are active during both bud development and flowering on rabbiteye and southern highbush blueberry plantings and are also excellent at parasitizing the larvae concealed within the blueberry buds where chemical control cannot reach (Lyrene and Payne 1997). In high densities, they can kill as many midges as insecticides can (Sampson et al. 2013) and have been known to parasitize between 25%–40% of midge larvae (Roubos and Liburd 2013). This is due to the parasitoids’ ovipositional behavior, in which the females inject their eggs into already developing midge larvae well-hidden within the buds. The parasitoids develop inside the midge larvae, and adult parasitoids emerge instead of larvae (Sampson et al. 2013). They essentially kill their hosts but do not damage the blueberry buds in the process.
The most recognized micro-wasp families that protect blueberries against BGM include Platygastridae and Eulophidae. Platygaster, Synopeas, and Inostemma are the genera within Platygastridae best known to parasitize this pest. In an organic blueberry farm in north-central Florida, key parasitoid densities were sampled from early February to early March of 2022. Out of the four groups, Platygaster was the most numerous, consisting of 89% of the Eulophidae and Platygastridae present in the system (Figure 10). Selectively use reduced-risk insecticides to preserve their populations. When used correctly, reduced-risk insecticides applied before the bloom period can effectively decrease BGM, and any remaining survivors can be eliminated by these parasitoids (Grover 1986; Sampson et al. 2002).
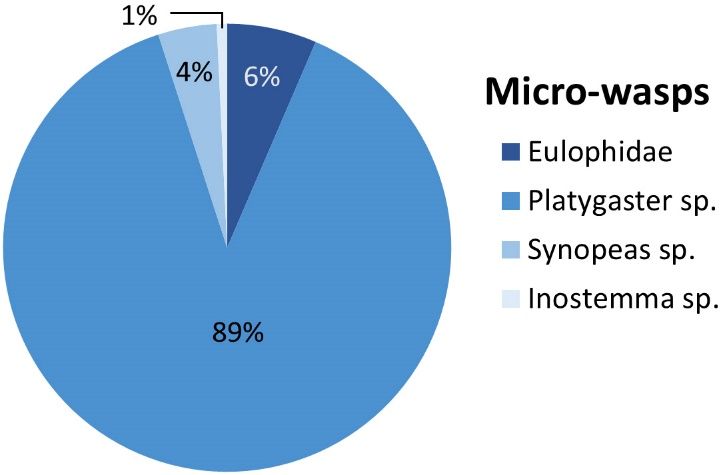
Credit: M. Lopez, UF/IFAS
Reduced-Risk Insecticides
The use of targeted insecticides in field IPM programs enhances the protection of biological control agents (Biddinger et al. 2014). This approach can help decrease reliance on chemical control methods, as well as delay the development of pest resistance and resurgence in subsequent generations (Gardner et al. 2011; Gentz et al. 2010). Reduced-risk insecticides should be considered due to their ability to synchronize with naturally occurring micro-wasps (parasitoids), providing effective control even before cecidomyiid pests establish themselves in the system. By utilizing reduced-risk insecticides, the risks of neurotoxic exposure to non-target organisms are minimized, and resistance development in the target pest is reduced (Peach et al. 2012; EPA 2023; Joshi et al. 2020).
Field Efficacy Study
In a field study conducted from February to March 2020, several reduced-risk insecticides were evaluated for their efficacy on BGM and their key parasitoid. The study included a conventional standard Malathion and a no-application control (Table 2). The parasitoids included important genera from the families Platygastridae and Eulophidae.
Results
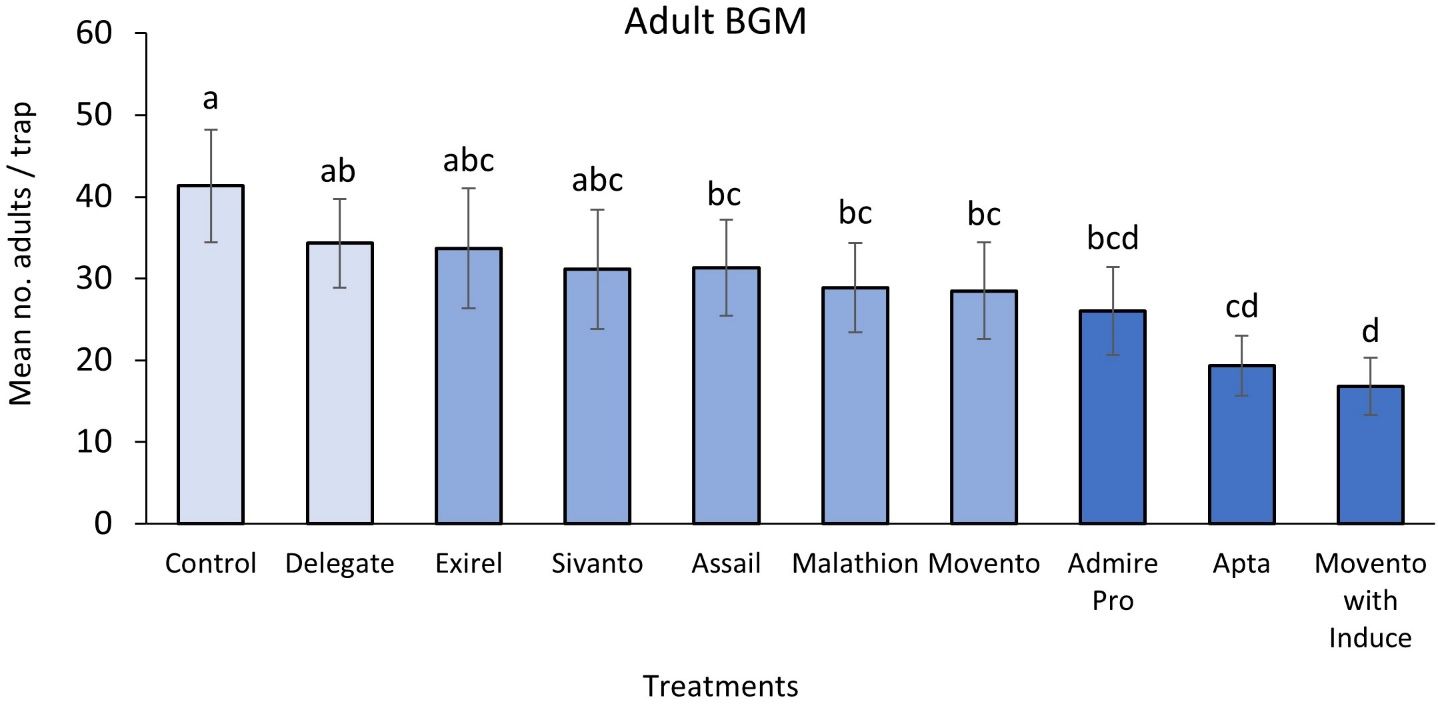
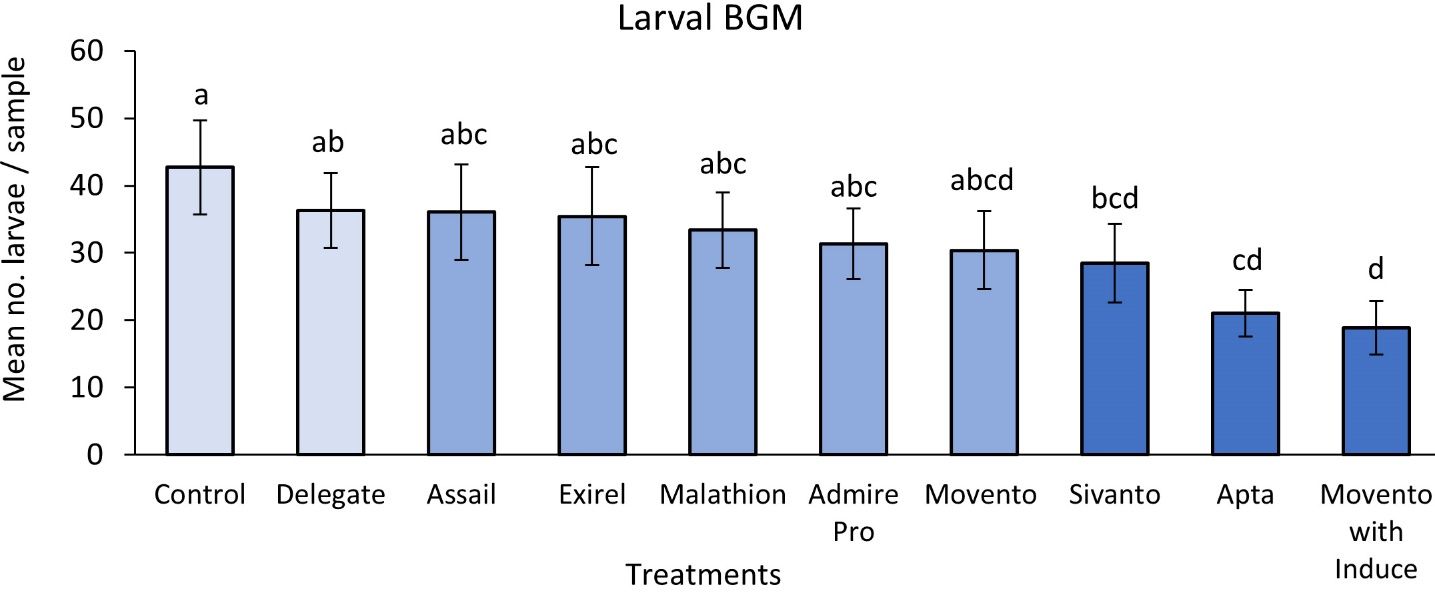
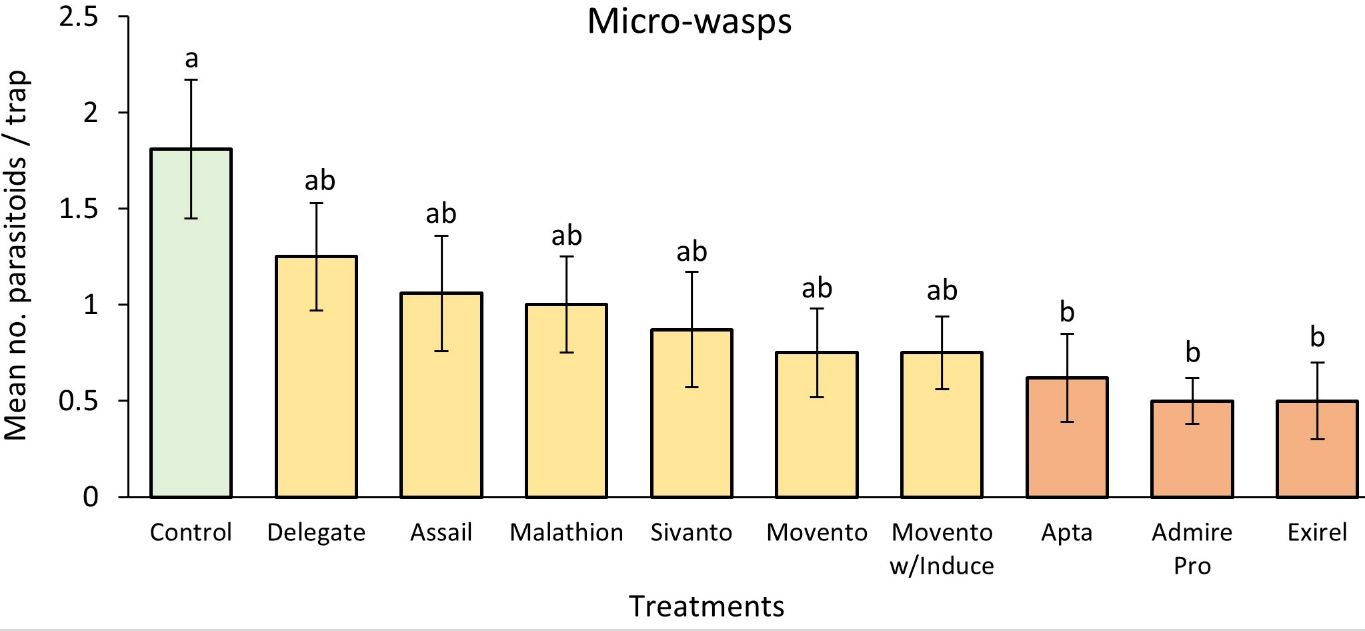
The results of the study indicated that Movento® (spirotetramat) with Induce® (nonionic low foam wetter/spreader adjuvant) was able to control 59% of BGM adults in the system (Figure 11) and 56% of BGM larvae hidden within the blueberry buds (Figure 12). Movento®’s mode of action is especially effective to target midges hidden deep within floral and leaf buds, which are impossible to reach using insecticides with different modes of action. The results also showed that Movento® with Induce® did not significantly reduce parasitoid numbers (Figure 13).
Credit: M. Lopez, UF/IFAS
Credit: M. Lopez, UF/IFAS
Credit: M. Lopez, UF/ IFAS
Apta™ (tophenpyrad) was the second best against BGM, reducing adults by 53% (Figure 11) and larvae by 51% (Figure 12). Both Apta™ and Movento® with Induce® also performed significantly better than Delegate®. Note that Apta™ was one of the three most lethal to the micro-wasp parasitoids— the first being Exirel® (cyantraniliprole) followed by Admire® Pro (imidacloprid) (Figure 13).
The least effective insecticide against BGM was Delegate® (spinetoram), having 15% and 17% efficacy against larvae and adults, respectively. Subsequently, Delegate® was also the least toxic to the key parasitoids of BGM (Figure 13).
Methodology
The experiment took place during the bud-break stage of floral bud development (Figure 3). It followed a completely randomized block design (CRBD) with four replicates (blocks). Each block was separated by a 19-meter-wide buffer zone in the east-west direction and a 7–6-meter-long buffer zone in the north-south direction.
Insecticides were applied at the recommended field rates, based on the level of BGM pressure, and the applications were performed in the morning or early evening to minimize contact with honeybees and other pollinators. The first application was conducted during the early bud break stage, followed by a second application ten days later. Sampling for adult BGM and their key parasitoids was performed by placing a clear sticky trap (Figure 5) in the middle to lower canopy of a central blueberry bush in each replicate block. Destructive sampling of floral buds involved randomly collecting 25 buds from three bushes in each replicate.
To learn more about the description, life history, damage, and monitoring of BGM, refer to the EDIS Publications "Blueberry Gall Midge on Southern Highbush Blueberry in Florida" (Liburd and Phillips 2019) and “Blueberry Gall Midge, Dasineura oxycoccana (Johnson) (Insecta: Diptera: Cecidomyiidae)” (Steck et al. 2020).
Table 2. List of insecticides tested against BGM with accompanying active ingredients, IRAC groups, and chemical classes.
References
Biddinger, D. J., T. W. Leslie, and N. K. Joshi. 2014. “Reduced-Risk Pest Management Programs for Eastern U.S. Peach Orchards: Effects on Arthropod Predators, Parasitoids, and Select Pests.” Journal of Economic Entomology. 107 (3). 1084–1091. https://doi.org/10.1603/EC13441
Bosio, G., C. Bogetti, G. Brussino, F. Gremo, and F. Scarpelli. 1998. “Dasineura oxycoccana, A New Pest of Blueberry in Italy.” Informatore Fitopatologico. 11: 36–41.
Dernisky, A. K., R. C. Evans, O. E. Liburd, and K. MacKenzie. 2005. “Characterization of Early Floral Damage by Cranberry Tipworm (Dasineuraoxy coccana Johnson) as a Precursor to Reduced Fruitset in Rabbiteye Blueberry (Vaccinium ashei Reade).” International Journal of Pest Management. 51(2): 143–148. https://doi.org/10.1080/09670870500130980
Gardner, J., M. P. Hoffmann, S. A. Pitcher, and J. K. Harper. 2011. “Integrating Insecticides and Trichogramma ostriniae to Control European Corn Borer in Sweet Corn: Economic Analysis.” Biological Control. 56 (1) 9–16. https://doi.org/10.1016/j.biocontrol.2010.08.010
Gentz, M. C., G. Murdoch, and G. F. King. 2010. “Tandem Use of Selective Insecticides and Natural Enemies for Effective, Reduced-Risk Pest Management. Biological Control. 52 (3) 208–215. https://doi.org/10.1016/j.biocontrol.2009.07.012
Grover, P. 1986. “Integrated Control of Midge Pests.” Cecidologia-Internationale. 7: 1–28.
Hahn N. G. Isaacs R. 2012. “Distribution and Phenology of Dasineura oxycoccana (Diptera: Cecidomyiidae) in Michigan Blueberries.” Environmental Entomology. 41: 455–462. https://doi.org/10.1603/EN12002
Insecticide Resistance Action Committee (IRAC). 2018. “The IRAC Mode of Action Classification Online.” 9.1. https://irac-online.org/modes-of-action/
Joshi, N. K., T. Leslie, E. G. Rajotte, and D. J. Biddinger. 2020. "Environmental impacts of reduced-risk and conventional pesticide programs differ in commercial apple orchards, but similarly influence pollinator community.” Chemosphere. 240. 124926. https://doi.org/10.1016/j.chemosphere.2019.124926
Liburd, O. E., and D. Phillips. 2019. “Blueberry Gall Midge on Southern Highbush Blueberry in Florida.” IN1239/ ENY-997. EDIS. https://edis.ifas.ufl.edu/pdffiles/IN/IN123900.pdf
Lopez, M. Forthcoming. “Development and Application of a Management Program for Blueberry Gall Midge in Florida’s Blueberry Plantings.” PhD diss., University of Florida.
Lyrene, P. and J.A. Payne. 1992. “Blueberry Gall Midge: A Pest on Rabbiteye Blueberry in Florida.” Proceedings, Florida State Horticultural Society. 105: 297–300.
Lyrene, P. M. and J.A. Payne. 1995. “Blueberry Gall Midge: A New Pest of Rabbiteye Blueberries.” Journal of Small Fruit and Viticulture. 2-3: 111–124. https://doi.org/10.1300/J065v03n02_12
Peach, D. A. H., J. T. Huber, and S. M. Fitzpatrick. 2012. “Hymenopterous Parasitoids of Cranberry Tipworm (Diptera: Cecidomyiidae) in British Columbia, Canada.” The Canadian Entomologist. 144(3): 487–490. https://doi.org/10.4039/tce.2012.37
Phillips, Doug, Jeffrey Williamson, James Olmstead, and Paul Lyrene. 2020. “Reproductive Growth and Development of Blueberry: HS976/HS220, Rev. 3/2020”. EDIS 2020 (2). https://doi.org/10.32473/edis-hs220-2020
Rhodes, E. M., N. D. Benda, and O. E. Liburd. 2014. “Field Distribution of Dasineura oxycoccana (Diptera: Cecidomyiidae) Adults, Larvae, Pupae, and Parasitoids and Evaluation of Monitoring Trap Designs in Florida.” Journal of Economic Entomology. 107 (1). 310–318. https://doi.org/10.1603/EC13409
Roubos C. R. Liburd O. E. 2010. “Evaluation of Emergence Traps for Monitoring Blueberry Gall Midge (Diptera: Cecidomyiidae) Adults and within Field Distribution of Midge Infestation.” Journal of Economic Entomology. 103(4): 1258–1267. https://doi.org/10.1603/EC09317
Roubos, C. R., and O. E. Liburd. 2013. “Parasitism of Dasineura oxycoccana (Diptera: Cecidomyiidae) in North Central Florida.” Environmental Entomology. 42(3): 424–429. https://doi.org/10.1603/EN12307.
Sampson, B. J., C. R. Roubos, S. J. Stringer, D. Marshall, O. E. Liburd. 2013. “Biology and Efficacy of Aprostocetus (Eulophidae: Hymenoptera) as a Parasitoid of the Blueberry Gall Midge Complex: Dasineura oxycoccana and Prodiplosis vaccinii (Diptera: Cecidomyiidae).” Journal of Economic Entomology. 106 (1) 73–79. https://doi.org/10.1603/EC12404
Sampson, B. J., S. J. Stringer, J. M. Spiers. 2002. “Integrated Pest Management for Dasineura oxycoccana (Diptera: Cecidomyiidae) in Blueberry.” Environmental Entomology. 31 (2): 339–347.
Spiers, J. M. 1978. “Effect of Stage of Bud Development on Cold Injury in Rabbiteye Blueberry.” Journal of the American Society for Horticultural Science. 103(4): 452–455. https://doi.org/10.21273/JASHS.103.4.452
Steck, G. J., P. M. Lyrene, and J. A. Payne. 2000. “Blueberry Gall Midge, Dasineura oxycoccana (Johnson) (Insecta: Diptera: Cecidomyiidae).” DPI Entomology Circular 373. Publication EENY136. http://entnemdept.ufl.edu/creatures/fruit/blueberry_gall_midge.htm
United States Environmental Protection Agency (EPA). 2023. “Conventional Reduced-Risk Pesticide Program.” https://www.epa.gov/pesticide-registration/conventional-reduced-risk-pesticide-program