Nematodes in Cotton Production
Nematodes are non-segmented roundworms that are generally microscopic. They live in animal hosts, soil/plant roots, or water. Nematodes in agricultural systems usually live in soil and can be divided into three categories: (1) entomopathogenic nematodes that infect insects; (2) free-living nematodes that feed on bacteria, fungi, or other nematodes and may be beneficial for crop production; and (3) plant-parasitic nematodes that feed only on plants and may drastically suppress crop yield. This article is a guide to managing plant-parasitic nematodes in cotton production and is intended for use by agricultural professionals involved in the cotton industry through production, processing, advising, research, or regulation.
Plant-parasitic nematodes of cotton reduce yield by decreasing root size and efficiency, leading to shorter shoots. Infected plants are generally smaller and show varying degrees of yellowing, called chlorosis. They may produce fewer or smaller bolls, and, in severe cases, they die. Nematode infection can also increase the incidence of fungal root diseases, particularly Fusarium wilt. The amount of damage nematodes cause is related to their population densities. The greater the density, the greater the damage.
Most plant-parasitic nematodes of cotton complete their life cycles (egg, four pre-adult juvenile stages, egg-producing adult) in two to four weeks depending on the nematode species and environmental conditions, so nematodes may go through six or more generations in a single growing season. A mature female nematode can produce upwards of hundreds of eggs, depending on the nematode species and environmental conditions, so nematode populations can increase rapidly.
Plant-parasitic nematodes of cotton spend their entire life in soil or roots and can be classified by where they reside when feeding, something that is important when sampling for nematodes. Ectoparasites (ecto means outside) spend their entire lives outside the roots. Only the head end or stylet of an ectoparasite enters the roots when it feeds (Figure 1). (A stylet is a needle-like mouthpart that all plant-parasitic nematodes possess.) Endoparasites (endo means inside) enter the root as a juvenile or adult and remain in the root to feed. Some endoparasites are mobile while feeding (migratory endoparasite) while others do not move once they begin feeding (sedentary endoparasite). A portion of the population of endoparasitic nematodes can be found in the soil at any given time because eggs generally hatch in the soil and mobile nematode stages may move freely in soil or from root to root.
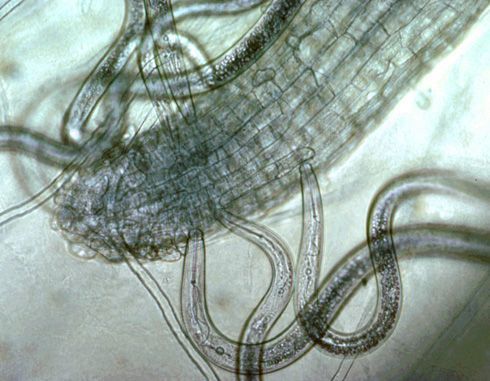
Credit: Ole Becker, University of California, Riverside. Used with permission
In Florida, southern root-knot nematode (Meloidogyne incognita), reniform nematode (Rotylenchulus reniformis), and sting nematode (Belonolaimus longicaudatus) cause major damage to cotton (Figures 2, 3, 4, 5, and 6). Southern root-knot nematode (a sedentary endoparasite) is probably the most common among these nematodes. It occurs in a variety of soil types and textures although it favors sandy soils. There are many species or types of root-knot nematodes, and it is important to distinguish between these types because different types infect different crops. Some species are also divided into different races based on the hosts they infect. Races 3 and 4 of southern root-knot nematode infect cotton, but races 1 and 2 do not. The invasive guava root-knot nematode (Meloidogyne enterolobii) also infects and damages cotton. While this nematode is prevalent in certain commodities and regions in Florida, it has not been detected in Florida cotton production yet. Guava root-knot nematode can overcome available resistance to root-knot nematodes in cotton cultivars and may have higher damage potential than endemic root-knot nematodes. For these reasons, vigilance for this invasive nematode is needed.

Credit: Charles Overstreet, Louisiana State University. Used with permission
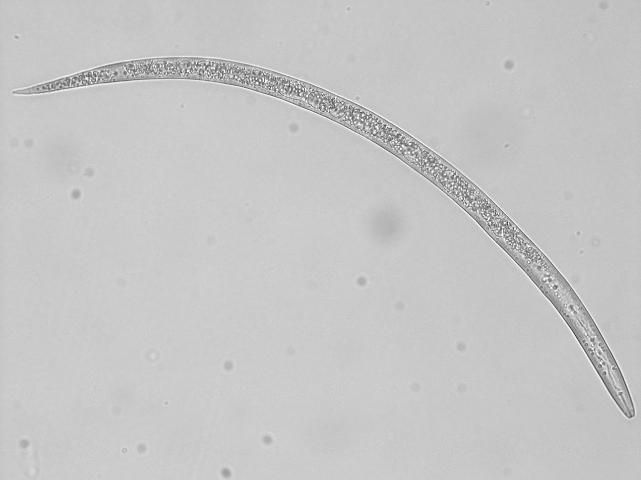
Credit: Zane Grabau, UF/IFAS

Credit: Charles Overstreet, Louisiana State University. Used with permission
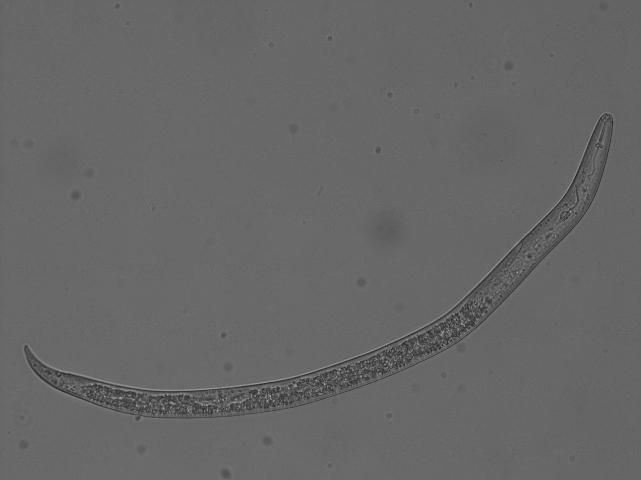
Credit: Zane Grabau, UF/IFAS
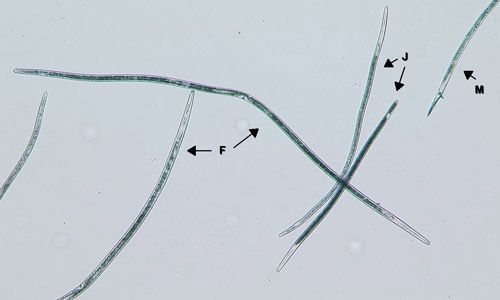
Credit: William Crow, UF/IFAS
Reniform nematode (a sedentary endoparasite) prefers fine-textured soils (around 70 percent sand or less), which probably restricts its distribution somewhat, but it is relatively common in cotton-growing regions of Florida. Root-knot and reniform nematode have moderate to high damage potential and are a major concern because they can increase rapidly in a field, particularly reniform nematode. Sting nematode (an ectoparasite) can be very damaging but is restricted to very sandy soils (80 percent or greater sand and 10 percent or less clay) with minimal organic matter, which limits its range. See the UF/IFAS publications on sting nematode and reniform nematode for more information about these nematodes. Columbia lance nematode (Hoplolaimus columbus) can be damaging to cotton, but it is apparently not common in Florida. Lesion nematodes (Pratylenchus spp.) are common and some species may parasitize cotton, but probably do not cause major damage.
Sampling for Nematodes
Determining what nematodes are present in a field and at what densities is helpful for developing a plan for managing nematodes. This can be accomplished by submitting soil or root samples to a professional nematology diagnostic lab such as the UF/IFAS Nematode Assay Laboratory. These samples may be submitted to aid with the diagnosis of disease problems or for advisory service to predict whether or not a potential nematode problem may exist for a future crop. This information allows growers the opportunity to implement management practices to reduce the damage that nematodes may cause.
Detailed information about sampling for nematodes can be found at the UF/IFAS nematode assay laboratory website. When submitting samples for testing for nematodes, either soil or both soil and plant root samples should be included. It is recommended to always collect soil because ectoparasitic nematodes can only be recovered from the soil and at least one stage of all endoparasites can be found in soil. It can be useful to collect plant roots in addition to soil when diagnosing crop damage. If the problem is caused by an endoparasitic nematode, then some nematodes will be found inside the roots.
Soil samples for nematodes should be taken to about 12 inches deep. If plants or remnants of plants are still in the field, soil samples should be taken within a few inches of plant stems and intersect plant roots if possible. Because nematode densities vary considerably across a field, about 20 soil cores of about an inch in diameter should be taken from an area of 10 acres or less. Thoroughly mix cores within a single area and submit a 1-pint portion of this mixture for analysis. For larger areas, take multiple, separate samples, making sure to properly label samples. Do not take samples when soil is excessively wet or dry. Store soil samples in closed plastic bags to protect them from drying and keep them cool but not frozen until shipment. When digging root samples, be sure to retain the soil surrounding the roots to slow decay by microbes. Root samples should be collected and stored in a similar manner to soil samples. If samples are intended to diagnose a current crop problem, it may be useful to collect separate samples from the diseased area and a healthy area for comparison.
While samples can be taken at any time of the year, nematode populations fluctuate throughout the year, so timing can be important. In most cases, nematode population densities peak around harvest while plant roots are still in the ground, so that is an ideal time to take routine or predictive samples. When diagnosing crop damage, take samples as soon as you see damage and again around harvest. For questions about sampling for nematodes, contact your local UF/IFAS Extension agent, personnel of the Nematode Assay Lab, or the author of this paper.
Foliar Symptoms
Symptoms of nematode infection in cotton are often indistinct and difficult to detect, so routine sampling for nematodes is especially important. Foliar symptoms of nematode infection are often similar to symptoms of nutrient deficiency or diseases and include stunted plants, sometimes with red or yellow hue (Figure 7). These symptoms often occur in oval or irregular patches in the field, corresponding to areas of greater plant-parasitic nematode densities (Figure 8). These patches may surround the initial entry point for the nematodes or correspond to uneven environmental conditions, such as soil type.

Credit: Zane Grabau, UF/IFAS

Credit: Lesley Schumacher, UF/IFAS
Plants infected with root-knot nematode also tend to be more prone to wilting (Figure 9). Sting nematode infection can cause severe stunting leading to reduced plant stand, symptoms that tend to appear early in the year (Figure 10). In fields infested with sting nematode, there may be sharp boundaries between healthy and diseased plants with apparently healthy plants next to severely stunted ones. Fields recently infected by reniform nematode may exhibit patchy stunting—less severe than sting nematode–but this aggressive nematode is often evenly distributed throughout a field after a few years, which leads to uniform stunting that is difficult to detect (Figure 11). At harvest time, nematode-infested areas may be recognized by slight stunting and reduced boll load (Figure 12).

Credit: Zane Grabau, UF/IFAS

Credit: Zane Grabau, UF/IFAS

Credit: Zane Grabau, UF/IFAS

Credit: Zane Grabau, UF/IFAS
Belowground Symptoms
Stunted root systems and reduced yield are common, generalized symptoms of plant-parasitic nematode infection. Specific belowground symptoms vary by nematode. Root-knot nematode infection is characterized by irregular swellings of the roots called galls (Figure 13). These galls are caused by an increase in the size and number of cells triggered by root-knot nematode feeding. Galls contain one or more sedentary adult female root-knot nematodes. These nematodes are contained in the roots and small, about 1 mm in diameter, so it is very difficult to view them in the field. However, with some practice, or the aid of a nematologist, it is possible to excise these pearly white females from the roots and look at them—a hand lens will make the examination much easier. Galls caused by root-knot nematode are often smaller on cotton than other crops. Gall size will vary based on the severity of the infection; galls may coalesce across the root surface if nematode densities are very great. Galls sometimes rot because they become entry points for secondary infection by other pathogens.

Credit: Zane Grabau, UF/IFAS
Soil may cling more easily to roots infected by root-knot or reniform nematode than uninfected roots. This is because female root-knot and reniform nematodes exude gelatinous egg masses—sac-shaped structures containing hundreds of nematode eggs. Reniform nematode produces these egg masses at the root surface while root-knot nematode may produce them inside or on the surface of the root. Soil adheres to the roots coated with moist egg masses. Egg masses are brown and about 1 mm or smaller, so they are not readily visible with the naked eye.
Reniform nematode does not induce root galling or any other distinct below-ground symptoms. Because above-ground symptoms of reniform nematode infection are also indistinct, it is especially important to soil sample regularly for this nematode. Sting nematode causes dark lesions of necrotic root tissue, often near the root tip where this nematode tends to infect. Lateral roots may proliferate above this point, resulting in severely stunted roots with a hairy or bearded appearance (Figure 14).

Credit: William Crow, UF/IFAS
Management
Once plant-parasitic nematodes infest a field, it is not possible to eradicate them completely. Rather, the goals are to minimize crop damage by nematodes and keep nematode densities low, ideally at a level where no crop loss occurs. The best way to manage a nematode problem is to use a combination of the most effective and economical practices for the given production situation based on nematode infestation level, other pest problems, available equipment, economics, and other considerations.
Exclusion
Exclusion is taking steps to stop or slow the spread of one or more plant-parasitic nematodes from infested to non-infested fields. Nematodes do not actively migrate from field to field; rather they are transported in infected soil, water, or plants. Wind and rain move these materials naturally, but they are also carried on field equipment. Besides avoiding intentionally moving soil or plant material, cleaning these materials from field equipment can slow nematode movement. This is particularly important when working both infested and non-infested areas. Nematodes can also be brought in on infected planting material. In general, this is not a large concern for row crops because cotton nematodes do not infect seeds, but closely monitor any crops that are started as transplants and rotated with cotton.
Crop Rotation
Producers can use crop rotation to reduce nematode densities by growing a crop that the particular nematode cannot reproduce on (a non-host crop) or that the nematode does not reproduce well on (poor host). In the absence of a host to reproduce on, the nematode population decreases as a portion of the nematodes die or are eaten. As a guide for choosing rotation crops for nematode management, the host statuses, based on current information, of selected cash and cover crops for the three major plant-parasitic nematodes of Florida cotton are listed in Table 1.
Root-knot and reniform nematodes have wide host ranges, but for each nematode there are a few agronomic or forage crops that can help with management. Cotton and peanut are good rotation partners for managing root-knot and reniform nematodes. Neither southern root-knot nor reniform nematode infects peanut, while the root-knot nematodes that infect peanut, Meloidogyne arenaria and Meloidogyne javanica (peanut and Javanese root-knot nematodes) do not infect cotton. Corn will help manage reniform nematode but is a host for most root-knot nematodes. Soybeans are good hosts for both root-knot and reniform nematodes.
Sting nematodes have a very wide host range, including cotton, peanut, and soybeans, so there are few rotations that will help manage these nematodes. Grasses are especially good hosts for sting nematodes and should be avoided where these nematodes are a concern. Weed management is an important supplement to crop rotation because plant-parasitic nematodes can be maintained or increased on weedy hosts, including volunteer cotton, growing in a non-host crop.
Fallow and Cover Cropping
Offseason periods when a cash crop is not grown should also be considered as part of a crop rotation strategy. These periods are opportunities to either reduce or worsen a nematode problem. Fallowing fields in the offseason can help reduce nematode densities, but only if weeds, including volunteer cotton, are controlled because many of them serve as hosts for nematodes. Growing an appropriate cover crop is usually best because erosion can be a major problem when fallowing fields, particularly on sandy soils.
If cover crops are grown, choosing a non-host for the nematodes present in a given field is critical for nematode management. Table 1 includes a summary of the host status of selected cover crops for root-knot and sting nematodes. Small-grain cover crops, such as wheat, rye, and oats, are poor or non-hosts for reniform nematode but good hosts of sting nematode. Susceptibility of these cover crops to southern root-knot nematode tends to vary by cultivar.
Some cover crops—such as Brassicas (radish, mustards, etc.)—may have nematicidal properties, directly reducing densities of nematodes. However, most cover crops with nematicidal properties are not suitable or have not been tested as a winter cover crop in Florida. Susceptibility to nematodes should always be considered when selecting a cover crop, even one with nematicidal properties, because these cover crops can also be good hosts for some plant-parasitic nematodes, such as most Brassicas for root-knot nematodes. For further information on cover cropping for root-knot nematode management, see (https://edis.ifas.ufl.edu/publication/in892).
Resistant Cultivars
Resistant cultivars prevent or reduce reproduction by one or more specific types or genera of plant-parasitic nematodes. Resistant cultivars can be categorized by their efficacy, which ranges from immune (no individuals of the target nematode can reproduce on the cultivar) to moderately resistant (some individuals can reproduce on the cultivar). Resistance is a trait that is selected for or bred in a cultivar; the given nematode can infect most cultivars of the crop to which the resistant cultivar belongs. Resistant cultivars sustain little or no damage from the target nematode, and growing resistant cultivars reduces field nematode population densities in a similar manner to non-host crops.
Some cotton cultivars with moderate to good resistance to root-knot nematode are commercially available. Most recent cotton cultivars with resistance to root-knot nematodes have “double-gene” resistance, which is more effective than older cultivars with "single-gene" resistance. Beginning in 2021, cotton cultivars with good resistance to reniform nematode are also available and typically also have resistance to southern root-knot nematode. Resistance to sting nematode is not documented in cotton. Using cotton varieties resistant to Fusarium wilt may curb yield loss from nematodes because nematode infection increases Fusarium wilt incidence.
Chemical and Biological Nematicides
Nematicides are chemical products intended to reduce nematode densities. To protect workers, consumers, and the environment, nematicides must be used in a legal manner, as specified on the label, including on specified crops only. Cotton nematicides may be fumigants, broad-spectrum pesticides that move through soil as a gas, or non-fumigants, liquid or granular products that affect only nematodes and, for some products, insects or fungi. Ideally, nematicides reduce crop damage and nematode populations. However, nematicide application sometimes provides only early-season nematode control, protecting crops from some damage but not preventing nematode populations from rebounding to high population densities later in the season.
Because nematicide application, particularly fumigation, is a relatively high-cost practice and agronomic crops such as cotton are relatively low-value, growers should test the level of nematode infestation in their fields before choosing to apply nematicides. Because nematode distribution is often patchy, applying nematicides only to the regions of a field with high nematode infestation can be a cost-efficient strategy.
Nematicides registered for Florida cotton and their application rates and methods are summarized in Table 2. The chemical 1,3-dichloropropene is a fumigant product available for use on cotton and can be effective for managing nematode problems. This product is applied as a liquid that volatilizes once in soil, moving through the soil profile. 1,3-D is often applied in the row at less than the full label rate to provide an economic return. To ensure maximum efficacy, apply 1,3-D in a manner that maximizes fumigant movement through the soil profile and improves chances for contact with nematodes. This means injecting the fumigant to at least a 10- to 12-inch depth using a shank or other equipment, then sealing and compacting the soil.
Metam sodium and metam potassium are broad-spectrum fumigants that are also labeled for nematode management in cotton. Due to product cost, application of metam salts is typically not economically feasible in cotton production.
There are three chemical non-fumigant nematicides currently labelled for Florida cotton. Vydate C-LV (oxamyl) is a post-plant foliar spray intended to supplement preplant or at-plant nematicides or seed treatment. Fluopyram is a new active ingredient introduced in the last few years, currently formulated as the product Velum, applied as an in-furrow spray for nematode control in cotton. Fluopyram also has fungicidal activity. AgLogic 15GG (active ingredient aldicarb) is a granular applied in-furrow and also has insecticidal activity.
A couple of biologically based products are also available but have not been tested extensively on cotton in Florida. Majestene is a relatively new biological nematicide composed of dead Burkholderia bacteria. It can be injected into the soil or applied as an in-furrow or banded spray. Melocon WG contains live spores of the fungus Paecilomyces lilacinus. Because the product contains live organisms, it must be stored in a cool (70°F or less) location, not mixed with or exposed to other chemicals, and applied to moist soil. These organic nematicides have not been tested extensively in cotton, but University of Florida research trials in other crops suggest these products are generally not as effective as chemical options but can have some value for nematode management.
A variety of chemical characteristics affect nematicide efficacy including mobility and persistence in soil and uptake and spread of the chemical in the soil. These properties and relative efficacy of cotton nematicides are listed in Table 3. A chemical that moves relatively quickly through soil is desired so that it contacts nematodes in a broader area of soil. A more persistent nematicide will remain in soil longer and increase contact time with nematodes. Chemicals that are too persistent or mobile are not desired as they may leach too quickly through the soil or create problems with residues, respectively. Non-fumigant nematicides move through the soil in water, so water solubility is directly related to their capacity to move through soil. Nematicides also vary in their human toxicity (Table 3), which influences safety risks and the personal protective equipment required for handlers.
In addition to nematicides applied to soil or plants, several seed treatments (Aeris, Avicta, VOTiVO, COPeO and others) are labeled for use against nematodes. Most of these products are pre-coated on seeds and may be exclusive to a particular seed brand. Because seed treatments contain small amounts of product that are not distributed widely in the soil, they may protect from some early-season nematode damage but can only provide modest nematode control and yield increase. Seed treatments are usually relatively low cost, though, so a modest yield increase would provide a return on investment.
Other Practices
Practices that promote plant health may help plants better tolerate nematode infection even if they do not reduce nematode populations. This includes practices such as maintaining soil fertility and tilth, providing adequate water, and managing insects and diseases (see EDIS publication AGR-194 Cotton Cultural Practices and Fertility Management (https://edis.ifas.ufl.edu/publication/ag200). Good knowledge of environmental and biological properties of one's soil can also aid in making good nematode management decisions. As mentioned above, soil type and texture influence where nematode damage is likely to occur.
Some soils keep plant-parasitic nematode densities low despite a susceptible crop. This suppression often develops gradually over a period of time. Practical and tested methods for developing suppressive soils have not been established, but natural predators and pests of nematodes are one cause of nematode-suppressive soils. Recognizing suppressive soil by monitoring nematode densities and cropping history can help cut costs by eliminating unnecessary management practices.
Selected References
Bell, A. A., A. F. Robinson, J. Quintana, S. E. Duke, J. L. Starr, D. M. Stelly, X. Zheng, S. Prom, V. Saladino, O. A. Gutierrez, S. R. Stetina, and R. L. Nichols. 2015. "Registration of BARBREN-713 germplasm line of upland cotton resistant to reniform and root-knot nematodes." Journal of Plant Registrations 9:89–93.
Chen, Z., and D. Dickson. 1998. "Review of Pasteuria penetrans: Biology, ecology, and biological control potential." Journal of Nematology 30:313–340.
Crow, W. T. 2013. "Effects of a commercial formulation of Paecilomyces lilacinus strain 251 on overseeded bermudagrass infested with Belonolaimus longicaudatus." Journal of Nematology 45:223–227.
Davis, R. F., and R. C. Kemerait. 2009. "The multi-year effects of repeatedly growing cotton with moderate resistance to Meloidogyne incognita." Journal of Nematology 41:140 –145.
Desaeger, J. A., and T. T. Watson. 2019. "Evaluation of new chemical and biological nematicides for managing Meloidogyne javanica in tomato production and associated double‐crops in Florida." Pest Management Science 75:3363–3370. https://doi.org/10.1002/ps.5481
Gattoni, K., N. Xiang, K. S. Lawrence, W. Groover, S. Till, D. Dyer, M.N. Rondon, and M. Foshee. 2018. "Evaluation of cotton nematicide combinations and rates for reniform nematode management in northern alabama, 2017." Plant Disease Management Reports 12:N040. https://www.plantmanagementnetwork.org/pub/trial/PDMR/volume12/
Grabau, Z. J., and C. Liu. 2019. "Nematicide application for reniform nematode management in Florida cotton, 2018". Plant Disease Management Reports 13: N008. https://www.plantmanagementnetwork.org/pub/trial/PDMR/volume13/
Grabau, Z. J. 2018. "Nematode management at different Velum Total rates in North Florida cotton with nematicide-free seed, 2017". Plant Disease Management Reports 12: N015. https://www.plantmanagementnetwork.org/pub/trial/PDMR/volume12/
Kirkpatrick, T. L., and C. S. Rothrock (eds.) 2001. Compendium of cotton diseases. Second ed. The American Phytopathological Society, Saint Paul.
Moore, S. R., and K. S. Lawrence. 2012. "Rotylenchulus reniformis in cotton: Current methods of management and the future of site-specific management." Nematropica 42:227 –236.
Lawrence, G. W., and K. S. McLean. 2000. "Effect of foliar applications of oxamyl with aldicarb for the management of Rotylenchulus reniformis on cotton". Journal of Nematology 32:542–549.
Lawrence, K., C. Land, N. Xiang, J. Luangkhot, and C. Norris. 2016. "Cotton variety and nematicide combinations for reniform management in North Alabama, 2015". Plant Disease Management Reports 10:N010 https://www.plantmanagementnetwork.org/pub/trial/PDMR/volume10/
Oka, Y. 2010. "Mechanisms of nematode suppression by organic soil amendments—A review." Applied Soil Ecology 44:101 –115.
Robinson, A., R. Inserra, E. Caswell-Chen, N. Vovlas, and A. Troccoli. 1997. "Rotylenchulus species: Identification, distribution, host ranges, and crop plant resistance." Nematropica 27:127 –180.
Thomas, S., J. Schroeder, and L. Murray. 2005. "The role of weeds in nematode management." Weed Science 53:923–928.
Wang, K., R. McSorley, R. N. Gallaher, and N. Kokalis-Burelle. 2008. "Cover crops and organic mulches for nematode, weed and plant health management." Nematology 10:231–242.
Watson, T. T., and J. A. Desaeger. 2019. "Evaluation of non-fumigant chemical and biological nematicides for strawberry production in Florida". Crop Protection 117:100–107. https://doi.org/10.1016/j.cropro.2018.11.019.
Table 1. Host status of selected cash and cover crops for management of specific plant-parasitic nematodes of cotton.
Table 2. Nematicides labeled for use in Florida cotton production and labelled rates, timing, and application methods.1
Table 3. Characteristics of nematicides in Florida cotton.