Introduction
Many postharvest decay problems result from the ineffective sanitizing of packinghouse dump tanks, flumes and hydrocoolers. Even healthy looking products from the field can harbor large populations of pathogens, particularly during warm, rainy weather. Pathogens present on freshly harvested fruits and vegetables accumulate in recirculated water handling systems. When fruits and vegetables contact water containing pathogens, they often become infected and subsequently decay during shipping and handling.
Although many packers routinely add chlorine to their water handling systems, failure to follow the UF/IFAS guidelines for packinghouse water sanitation may greatly reduce the effectiveness of this treatment in reducing postharvest decay. The current recommendation is constant maintenance of 100 to 150 parts per million (ppm) of free chlorine and a pH in the range of 6.5 to 7.5 for all recirculated water. There is a good possibility that decay problems will arise during handling and shipping whenever product contacts recirculated water that is not maintained under these conditions. In this article, we outline principles for effectively using chlorine for water sanitation.
Forms of Chlorine
The main forms of chlorine used include sodium hypochlorite (NaOCl), calcium hypochlorite (Ca(OCl)2), and chlorine gas (Cl2). Sodium hypochlorite is often sold as 12 to 15 % solutions. Calcium hypochlorite usually is sold as a powder or tablets in formulations of 65%. However, it does not dissolve readily (especially in cold water) and undissolved particles can injure fruits and vegetables. To prevent this, first dissolve the powder or granules in a small amount of warm water before adding it to the tank. If using tablets for continuous, slow release of chlorine, ensure that the tablets are placed where water circulates well around them. Chlorine gas comes in pressurized gas cylinders and should be handled cautiously according to label instructions.
Factors Influencing Chlorine Activity
Water pH
When sodium hypochlorite is added to water, it forms sodium hydroxide (NaOH) and hypochlorous acid (HOCl). All three forms of chlorine produce hypochlorous acid (also called available chlorine or active chlorine). Hypochlorous acid is what kills pathogens. In high pH solutions, most of the hypochlorous acid disassociates to form hypochlorite ion (OCl-) which is not an effective sanitizer. Testing kits for free chlorine measure both hypochlorous acid and hypochlorite ion and alone do not indicate the quantity of available chlorine that kills pathogens. Chlorine solutions with pH above 8 are relatively ineffective against pathogens. Below pH 6, chlorine is more corrosive to equipment and activity is rapidly lost. A pH of around 7 will maintain about 80% of the chlorine in the available (hypochlorous acid) form with very little gas formed (Figure 1). Thus, in order to know the sanitizing strength of ones chlorine solution, both pH and free chlorine must be measured.
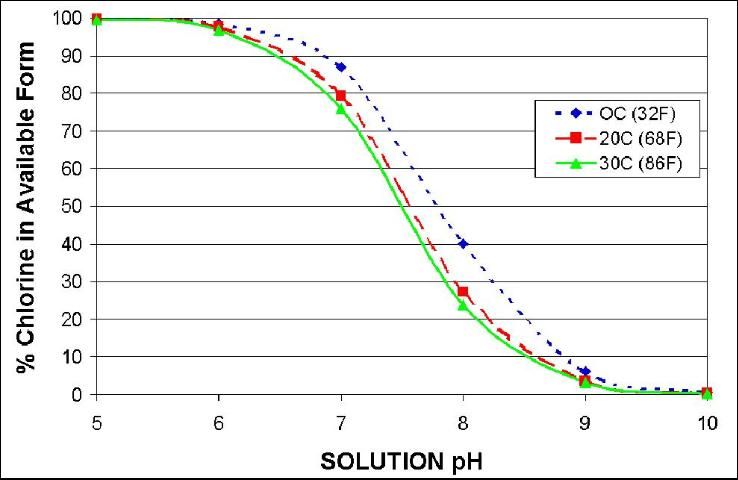
Both water source and form of chlorine used will affect pH management. Fresh water in Florida may have a pH above 8.0 due to dissolved calcium carbonate. Adding either sodium hypochlorite or calcium hypochlorite will increase pH, while adding chlorine gas will decrease pH. After adding commercial chlorine, adjust the pH of the water to 7 by adding either acid or base. Muriatic (HCl) or citric acid are commonly used to lower pH while sodium hydroxide (lye) will raise pH. The pH of water can be determined by using an electronic pH meter.
Chlorine Concentration
Although low concentrations of hypochlorous acid (< 40 ppm) have been reported to kill most pathogens within 1 minute, higher concentrations (100–150 ppm) are commonly used to compensate for various losses of available chlorine in the tank.
Exposure Time
High available chlorine concentrations kill pathogens after short exposure times (< 1 min.). At lower concentrations, more contact time is required to kill the pathogens.
Amount of Organic Matter in the Water (e.g. fruit, leaves, and soil)
Organic matter in the water will inactivate hypochlorous acid and can quickly reduce the amount of available chlorine. Chlorine which combines with organic matter is no longer active against pathogens but may still be measured by total chlorine testing kits.
Water Temperature
At higher temperatures, hypochlorous acid kills pathogens more quickly but is also lost more rapidly due to reactions with organic matter.
Type and Growth Stage of the Pathogens
Although germinating spores and mycelium are relatively easy to kill, spores are much more resistant to chlorine, and pathogens growing inside fruit and vegetable tissue (inside wounds or as quiescent infections) are shielded from the chlorine and not killed.
Maintaining Adequate Chlorine Concentrations
Chlorine must be continuously added to the water to replace chlorine lost to reactions with organic matter, chemicals, microorganisms, and the surfaces of fruits and vegetables. There are several ways to maintain adequate chlorine concentrations. Equipment is available to automatically measure chlorine concentrations and to add chlorine to the water when needed. Moreover, certain types of systems also automatically maintain the proper pH range. Automated dispensing of chlorine products requires frequent measurement of the chlorine concentration to verify proper operation. Managers must be vigilant with systems designed to dispense chlorine at a uniform rate because the chlorine demand can change abruptly, such as with the addition of product from a different field, a different grower, or a different field crew. Manual addition of chlorine products can be used if the manager is diligent in measuring and adjusting chlorine concentrations and pH. Measurements should be taken on at least an hourly basis. However, in our conversations with packinghouse managers around the state, it appears that manual water sanitation is usually less than adequate due to time constraints encountered during typical packing operations.
Chlorine Test Kits
Make certain the test kit measures free chlorine (not total) and be familiar with the concentration limits of the kit. Swimming pool-type kits usually measure in the range of 1 to 5 ppm free chlorine. These kits can provide accurate measurement if the water samples from the packinghouse system are diluted to the range of the kit before being tested. Distilled water should be used to dilute the sample for accurate determination of free chlorine. Diluting the sample with sulfur water or water containing organic matter or other chemicals will interfere with accurate measurements of free chlorine.
Recommendations
- Maintain free chlorine levels between 100 to 150 parts per million.
- Maintain pH between 6.5 and 7.5.
- Check free chlorine and pH levels frequently. Installation of automated systems to monitor and adjust chlorine and pH levels may be effective, but require regular calibration and maintenance.
- Drain the tank at the end of each day and refill with clean water.
- Use all chemicals according to their label instructions (e.g. chlorine, muriatic acid, lye, etc.).
- Use self-cleaning screens in dump tanks to remove large debris.
- Consult local regulations for disposal of chlorinated water.
Mixing Chlorine Solutions
Use table 1 as a quck guide for mixing chlorine solutions. To prepare a specific free chlorine solution (ppm) using sodium hypochlorite (NaOCl), use the following formula.
1. Determine amount of sodium hypochlorite (NaOCl) concentrate to be added to the total volume of water (units for NaOCl concentrate to add and total volume must be the same):
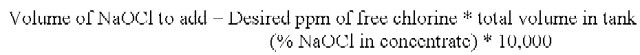
2. Add calculated amount of NaOCl concentrate to tank and bring up to final volume with water.
Example
To achieve a 150 ppm free chlorine concentration in a 1,000 gallon dump tank using a 12.75% sodium hypochlorite solution.
1.
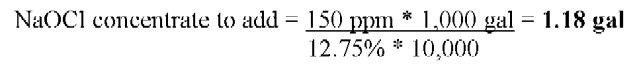
2. Add 1.18 gallons of 12.75% sodium hypochlorite to 998.82 gallons of water. Adjust pH to between 6.5 and 7.5.