Aglaonema and Dieffenbachia (members of the plant family Araceae) are popular tropical foliage plants. In order to develop new ornamental Aglaonema and Dieffenbachia cultivars, plant breeders must be able to overcome breeding barriers. Ability to control flowering, effect successful pollination and secure seed production are essential for hybridization of these crops.

Commonly known as dumb cane, the genus Dieffenbachia includes about 30 species native to the tropical forests of Central and South America. The chromosome number of most Dieffenbachia species is 2n = 34. Although some Dieffenbachia plants may cross-pollinate in the wild, directed interspecific hybridization by breeders is the primary means of generating new commercial cultivars.
Academic and private commercial breeding programs that develop Dieffenbachia have focused on novel leaf variegation patterns and on increased branching to give the plant a full appearance. Almost 100 cultivars have been introduced over the years, but only about 20 Dieffenbachia cultivars remain consistently popular commercially. Nine hybrids have been released through the University of Florida MREC-Apopka Plant Breeding Program including 'Triumph', 'Victory', 'Tropic Star', 'Starry Nights', 'Star White', 'Star Bright', 'Sparkles', 'Tropic Honey', and 'Sterling'.
When breeding for novel leaf patterns, inheritance of variegation is dominant over inheritance of non-variegation. A single dominant allele interacts with modifier genes to determine variegation patterns within Dieffenbachia. Multiple genes control basal shoot formation. For more information, the reader is referred to Henny 1988, Henny and Chen 2003, and Henny, et al 2004.

Commonly called Chinese evergreen, Aglaonema is one of the most widely used foliage plants due to its ability to tolerate low light and low humidity, and its resistance to diseases and pests. This crop has been commercially cultivated in Florida since the 1930s. The genus Aglaonema includes 21 species native to Southeast Asia. In the wild, Aglaonema species are open-pollinated. The base number of chromosomes is 2n = 6, with subsequent polyploidy in many cases.
Aglaonema hybrids for ornamental foliage production are almost exclusively developed via interspecific hybridization within traditional breeding programs. The species A. nitidum, A. commutatum, A. costatum, and A. rotundum are commonly used in the interspecific hybridization work. Current breeding activities focus on generating novel foliage variegation patterns, new petiole colors, increased branching, and better chilling resistance.
Popular hybrids from the breeding program at the University of Florida's MREC in Apopka include Aglaonema 'Silver Bay', which has a medium green leaf blade overlaid with a gray-green center; 'Emerald Bay', which has a white and green mottled stem; and 'Diamond Bay' displaying a bold central gray stripe against a dark green leaf blade. For more information, the reader is referred to Henny and Chen 2008; and Henny et al. 2003.
Flowering, Pollination and Seed Production
Under natural conditions, Aglaonema and Dieffenbachia produce only 3 to 5 flowers per stem per year. Different species within each genus may not flower simultaneously. This potential barrier to breeding has been overcome by the use of gibberellic acid (GA3) sprays to stimulate flowering. Treatment consists of a single foliar spray of 250 to 1,000 ppm GA3. Flowers appear 90 to 120 days after treatment. Additionally, GA3 treatment increases the number of flowers produced per plant. This helps to ensure a sufficient supply of flowers for breeding purposes. With careful planning, different species of the same genus can be induced to flower simultaneously. (Henny, 2001).
Both Aglaonema and Dieffenbachia have unisexual flowers (Figure 3 and Figure 4). Aroid inflorescences consist of a spadix enclosed by a spathe. The spadix is a fleshy spike covered with many small, unisexual flowers. The unisexual flowers contain male (staminate) flowers on the upper half of the spadix and female (pistillate) flowers on the lower portion of the spadix, with a small area between that may be devoid of flowers.
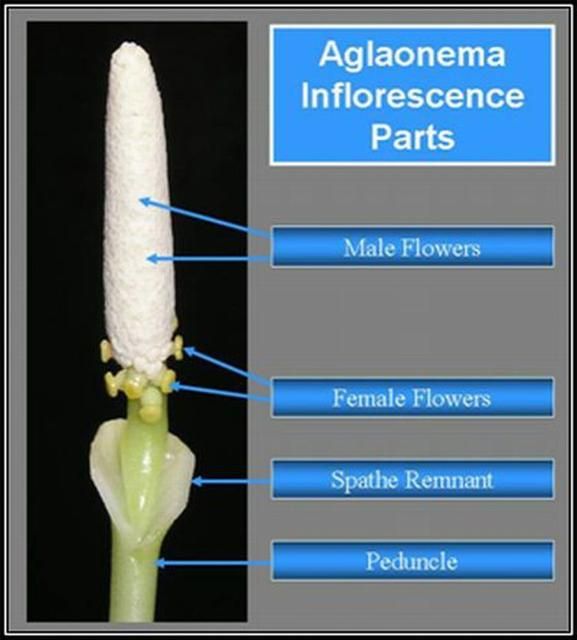
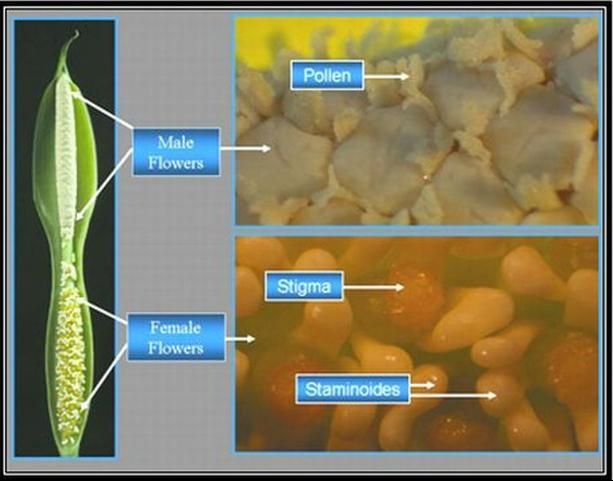
To prevent inbreeding in their native habitat, both genera are dichogamous (male and female receptivity is not synchronous). The inflorescences of Dieffenbachia and Aglaonema exhibit protogyny (female receptivity occurs first). Female flowers on the spadix mature first and simultaneously. Then, approximately 2 days later, after the females on that spadix are no longer receptive; the male flowers of that spadix mature simultaneously and produce pollen. This discourages self-pollination.
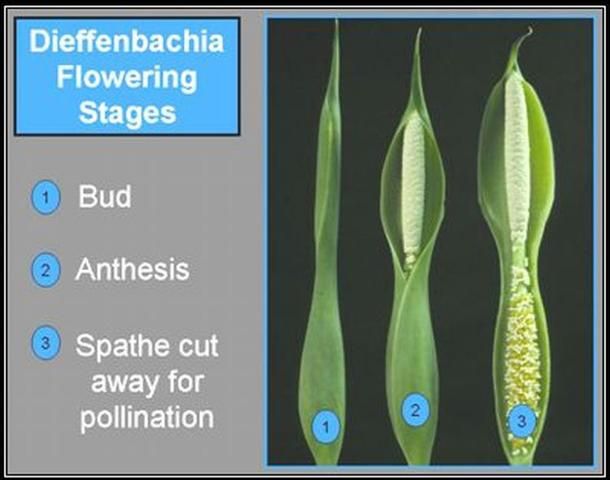
Receptivity of female flowers coincides with the unfurling of the spathe (Figure 5 and Figure 6). Spathes from these two genera normally begin to unfurl at night and pollination can occur any time during the following day. Receptivity of Aglaonema and Dieffenbachia flowers lasts at least 24 hours as evidenced by pollen germination studies. Female flower surfaces that have become discolored and soft are no longer receptive. Seed has been obtained from flowers of both genera pollinated one full day after spathe unfurling, but the number of seeds is smaller.

To cross-pollinate Aglaonema and Dieffenbachia, it is necessary to obtain pollen from one inflorescence with ripe males and to manually transfer the pollen to another inflorescence that has receptive females (Figure 7). The pollen is not wind disseminated. Pollen transfer begins by using a small, soft brush to sweep pollen into a container. The same brush used to collect the pollen may be used for application of pollen to the female. First make the brush sticky by gently wiping it against the female stigmatic surfaces. Then dip the sticky brush into the pollen supply and lightly brush pollen grains onto the stigmatic surfaces of receptive flowers.

If pollen is in short supply, it can be stored in a container in a high-humidity environment in a refrigerator. Humidity affects pollen viability (Figure 8.) Collect the pollen in a container such as a petri dish. Place the petri dish on top of a wet paper towel. Then enclose both the petri dish and the paper towel within a larger, sealed storage container. At no time should the pollen be directly in contact with the wet paper towel. Avoid splashing water droplets onto the pollen. Aglaonema and Dieffenbachia pollen is short-lived, and germination ability declines within 1 to 2 days of storage. It is always best to use fresh pollen.

Following pollination, Dieffenbachia flowers require 100 percent relative humidity for pollen to germinate. This can be done by wrapping the entire spadix with moistened paper toweling and enclosing it in a plastic bag. The wrap is removed the next day so that it will not interfere with pollen production on the upper portion of the same flower. Pollen germination in Aglaonema is greater when provided high humidity, but Aglaonema pollen is not as sensitive as Dieffenbachia pollen.

Pollinated Dieffenbachia flowers develop mature fruits within 4 to 5 months. Aglaonema fruits mature in 4 to 6 months, although some hybrids have taken up to 1 year to develop ripe fruit. In both genera the seed coat turns bright red when the seed is mature.

To enhance germination, separate the mature seeds from the spadix. This is to lessen the chance of disease contamination from decaying fruit. Both Dieffenbachia and Aglaonema have large seeds, and the fleshy seed covering should be removed from the red, berry-like fruit before planting the seed. Keep cleaned seeds moist and plant them before they become dry.
High germination is achieved if seed are sown on top of a moist potting medium containing up to 50 percent peat moss by volume, and covered with plastic to prevent drying. Aroid seed begin to grow as soon as they are sown. The medium should be kept at a minimum of 70°F. Seedlings should be transferred to individual pots after the first true leaves are produced. Most aroid seedlings require 1 year of growth before they are large enough to be evaluated.
Literature Cited
Henny, Richard J. 2001. Tips on Regulating Growth of Floriculture Crops. Foliage Plants Editor: Michelle, Gaston Columbus, Ohio: Ohio Florists Association, pp. 83-87.
Henny, R.J. 1988. Ornamental aroids: culture and breeding. In J. Janick (editor), Horticultural Reviews Vol. X., Timber Press, Portland, Or. Inc. p. 1-33.
Henny, R.J.and J Chen. 2008.New Plant Introductions Drive Markets https://edis.ifas.ufl.edu/EP357.
Henny, R.J. and J. Chen 2003. Foliage plant cultivar development. Plant Breeding Reviews 23:245-290.
Henny, R.J., D.J. Norman, and J. Chen. 2004. Progress in ornamental aroid breeding research. Annals of the Missouri Botanical Garden 91:465-473.