Introduction
Hops (Humulus lupulus) are a dioecious perennial plant belonging to the genus Humulus and native to the Northern Hemisphere. It is a vigorous climbing vine that can grow up to 7 to 8 meters (23 to 26 feet) in height with roots that can grow up to 100 meters (328 feet) in length. Three states in the United States, Washington, Oregon, and Idaho, are the major providers for hop cultivation due to optimum light and temperature conditions. Specifically, hop flowering is controlled by plant maturity and day length. Latitudes between 38° and 51° N are ideal for hop cultivation (Koetter 2010). Locations south of 38° have daylength too short for high yield of cones, whereas locations north of 51° often have severe cold weather that limits seasonal growth of hops.
Hops are prized for their female flowers or cones (Figure 1) that contain lupulin glands full of resins and essential oils responsible for the unique aroma and microbial stability of beer. Based on the National Hop Report in 2018, the production of hops increased from 87 million pounds in 2016 to 107 million pounds in 2018 (USDA 2018). In addition to brewing beer, the use of hops for medicinal purposes has gained momentum in recent years and attracted widespread attention. Therefore, it is estimated that the demand and production of hops will continue to increase within the next decade.

Credit: Tyler Jones, UF/IFAS
Vegetative Propagation versus Micropropagation
Commercial propagation of hops has traditionally been conducted using vegetative techniques, either through rhizome or herbaceous stem cuttings. Micropropagation, also called plant tissue culture production, is a modern technique that possesses the advantage of increased production rate with less time through multiplying plants in vitro. Micropropagation also has the advantage of reduced disease incidence compared to vegetative propagation and can allow for plant production without seasonal restrictions limiting plant growth. Lastly, micropropagation of hops can be used to produce and extract chemicals for pharmaceutical uses.
Micropropagation and Light-Emitting Diodes (LEDs)
Protocols for establishment of hops in tissue culture exist and are successfully applied commercially (Batista et al., 1996, 2000, 2008). Because micropropagation is a high-cost technique, in vitro rooting is usually excluded unless necessary, depending on the genotype. Thus, the main disadvantages of micropropagation are cost and successful plant establishment ex vitro.
Root development is essential for successful plant establishment after transplanting, especially from a highly controlled in vitro environment to the open greenhouses. When plants are propagated using tissue culture techniques, fluorescent and incandescent lamps are commonly used as the light sources. While light quality is an important environmental factor affecting plant growth and development, these light sources are generally of low quality for promoting growth. In contrast, light-emitting diode (LED) lamps can produce an engineered spectrum of various wavelengths beneficial to plant growth. Research examining the use of LED lights in plant production shows the ability to promote growth in plants through emission of higher light quality, such as enhanced red and blue wavelength discharge. This is particularly important and useful to stimulate biomass and root development (Gupta 2013). In addition to higher light quality, the longevity and efficiency of LED lights make them ideal for micropropagation facilities where supplemental lighting is used for extended periods of time.
To address the need for successful micropropagation of hops, this publication provides information to plant producers on the use and application of LED lights to enhance leaf area and root length of hops.
Hops Micropropagation under LED Light Experiment
Surface Sterilization and Micropropagation
Rhizomes of the hop variety Tettnanger were grown in the greenhouse (Apopka, FL) for two months. New growth with multiple internodes was surface sterilized by washing the explants under running water for 30 minutes. Afterward, explants were shaken with 1.3% hypochlorite acid and Tween20 (polysorbate-type surfactant) for 20 minutes and rinsed with sterilized water. Internodes were excised and placed in autoclaved containers prepared with 40 mL of regeneration media (4.4 g of Murashige and Skoog (MS) medium+30 g Sucrose+7 g Agar per Liter at pH 5.8). New shoots from the axillary buds were grown in tissue culture until they were 8 cm long before shoot tips were excised for treatments.
LED Light Treatments
The newly regenerated shoots in tissue culture were excised and placed on regeneration media, as described previously, and treated with and without 1 g/L of activated charcoal. Charcoal was added as an additional treatment due to its known function to improve plant cell growth and development in tissue culture (Thomas 2008). Treatments with and without charcoal were placed under fluorescent light (WL) or LED light for a duration of 16 hours light and 8 hours dark at constant room temperature for four weeks. Hubbell's NutriLED four-foot unit (62 watt) LED lights produced a red spectral output of 660 nm, blue spectral output of 460 nm, and a red to blue LED ratio of 2:1 (Hubbell 2019). Four replicates were used for each treatment with five shoots per replicate/container. The workflow layout is summarized in Figure 2.
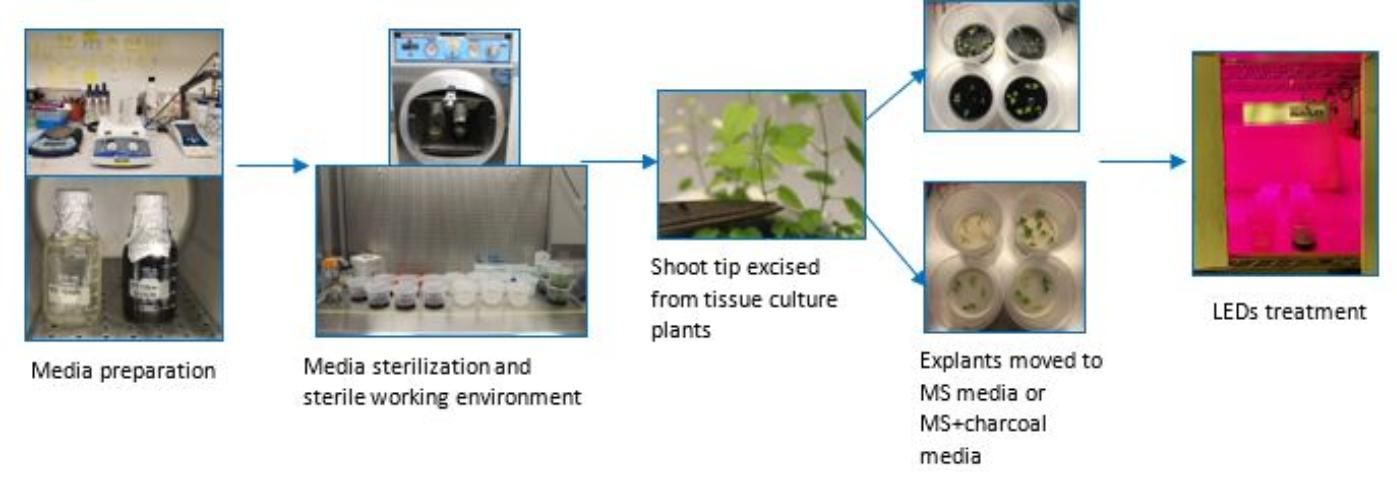
Credit: Chi Nguyen and Abigail Plontke, UF/IFAS
Micropropagation of "Tettnanger" under LED light increases root length and plant height
In vitro rooting is critical for plants produced through micropropagation because it helps plants survive the environmental condition changes from tissue culture to the greenhouse. However, the rooting phase increases production cost and is usually avoided or shortened in favor of ex vitro rooting. If plants could produce vigorous roots during the shoot regeneration phase in tissue culture, it would save time and lower production cost. Tettnanger's root length was longer under LED light treatment when compared to fluorescent light (WL). With the addition of activated charcoal, root length decreased significantly under white light and failed to grow under LED light (Figure 3B). Similar results can be seen for plant height between the treatments with plant height being the highest under LED light treatment without the charcoal and lowest with charcoal added to the media (Figure 3A).
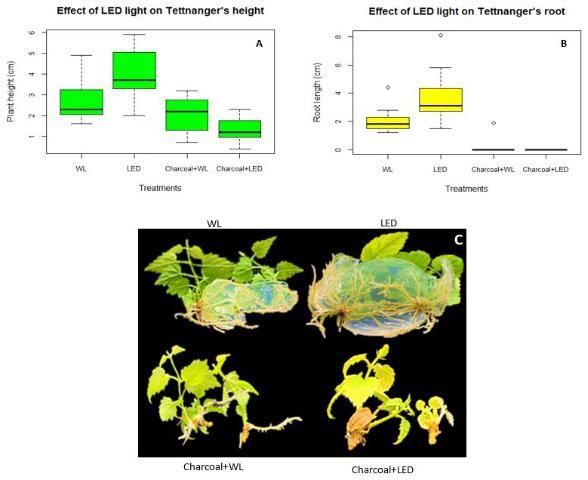
Credit: Chi Nguyen and Dominic Vu, UF/IFAS
Overall, supplemental LED light can increase both plant height and rooting of hops in tissue culture only on MS media. This is beneficial for timing and production of hops.
Micropropagation of "Tettnanger" under LED light increases leaf area
Leaf area growth determines light interception, which is an important indicator of plant productivity. Besides root length, leaf area was noticeably different for hops grown under white light and those cultivated under LED light. Leaf area of Tettnanger significantly increased when grown under LED light, but only on media without activated charcoal (Figure 4). Like plant height and root length, charcoal in media decreased leaf area when grown under both white light and LED light. An increase in leaf area is helpful for photosynthesis and promoting plant establishment ex vitro.
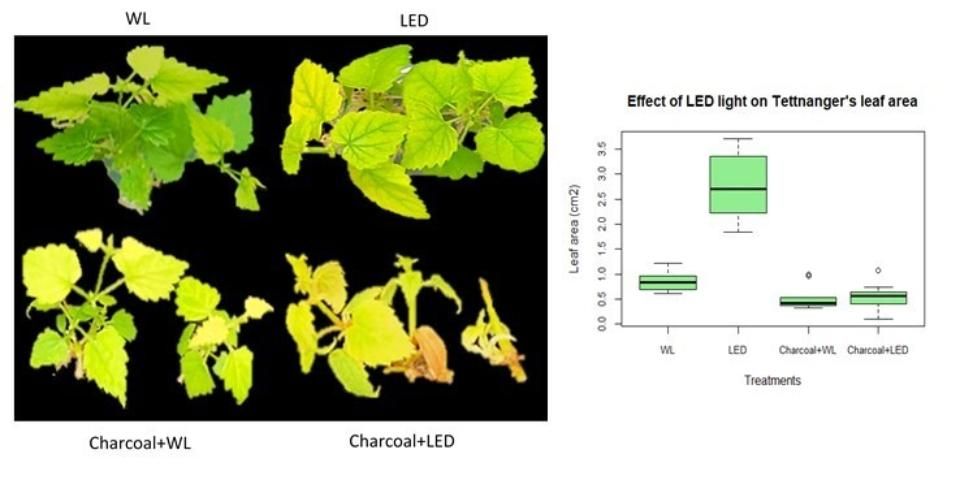
Credit: Chi Nguyen and Dominic Vu, UF/IFAS
Conclusion
Fluorescent lamps are usually the main source of light for commercial micropropagation of plants. However, they produce a wide range of wavelengths (350–750 nm) while consuming a high amount of power. In contrast, LED light can emit specific wavelengths, such as red and blue, that are used for photosynthesis. Additionally, the efficiency and cost-effectiveness of LED light makes it an attractive alternative. Given the popularity of hops in recent years, the experiment tested hops in vitro under LED light to provide plant producers insights to the effects of LED light on micropropagation of hops.
When the hop variety Tettnanger was examined under LED light versus fluorescent light (WL) in vitro, it was found that LED light promoted root and leaf development on MS media without activated charcoal. Although activated charcoal can be beneficial for micropropagation of certain plant species, it has been found to hinder the growth of hops under both fluorescent light and LED light. Thus, the effects of activated charcoal and LED light may be species dependent. Additionally, light combination and ratio are important factors when using LED light. For this specific experiment, both red (660 nm) and blue light (460 nm) were used with a ratio of 2:1. Different combinations and ratios of light, such as white supplemented with red and/or blue, have been reported in many species with altering effects. Thus, it is recommended to test different commercially manufactured LED lights to find the best combination for a specific plant species.
In summary, LED light can significantly increase hops growth and development in vitro only on MS media when compared to fluorescent light (WL).
References
Batista, D., L. Ascensão, M. J. Sousa, and M. S. Pais. 2000. "Adventitious Shoot Mass Production of Hop (Humulus lupulus L.) var. Eroica in Liquid Medium from Organogenic Nodule Cultures." Plant Science 151 (1): 47–57. https://doi.org/10.1016/S0168-9452(99)00196-X
Batista, D., S. Fonseca, S. Serrazina, A. Figueiredo, and M. S. Pais. 2008. "Efficient and Stable Transformation of Hop (Humulus lupulus L.) var. Eroica by Particle Bombardment." Plant Cell Reports 27 (7): 1185–1196. https://doi.org/10.1007/s00299-008-0537-6
Batista, D., M. J. Sousa, and M. S. Pais. 1996. "Plant Regeneration from Stem and Petiole-Derived Callus of Humulus lupulus L. (Hop) Clone Bragança and var. Brewers Gold." In Vitro Cellular Development & Biology - Plant 32 (1): 37–41. https://doi.org/10.1007/BF02823011
Goto, E. 2012. "Plant Production in a Closed Plant Factory with Artificial Lighting." VII Internat. Symposium on Light in Horticult. Syst. 956: 37–49.
Gupta, S. D., and B. Jatothu. 2013. "Fundamentals and Applications of Light-Emitting Diodes (LEDs) in In Vitro Plant Growth and Morphogenesis." Plant Biotechnol. Rep. 7 (3): 211–220.
Koetter, U., and M. Biendl. 2010. "Hops." HerbalGram 87: 44–57.
Premier Lighting. 2019. "Hubbell NutriLED – LED Horticultural Light." https://ledt8bulb.com/hubbell-nutriled-led-horticultural-light-fixture.html
Rodríguez-Vidal, E., D. Otaduy, D. Ortiz, F. González, F. Moreno, and J. M. Saiz. 2014. "Optical Performance of a Versatile Illumination System for High Divergence LED Sources." Optik 125 (5): 1657–1662. https://doi.org/10.1016/j.ijleo.2013.09.064
Thomas, T. D. 2008. "The Role of Activated Charcoal in Plant Tissue Culture." Biotechnol. Adv. 26 (6): 618–631. https://doi.org/10.1016/j.biotechadv.2008.08.003
USDA-NASS. 2018. "National Hop Report." In Agriculture USDA, edited by National Agricultural Statistics Service.
Wu, S. J., H. C. Hsu, S. L. Fu, and J. N. Yeh. 2014. "Numerical Simulation of High Power LED Heat-Dissipating System." Electron. Mater. Lett. 10 (2): 497–502.