Cause: Bacterial black spot is caused by the bacterium Xanthomonas axonopodis pv. mangiferaeindicae (Gagnevin and Pruvost 2001, Ah-You et al. 2009).
Background
South Florida has an estimated 1,351 acres of commercial mango production (Crane 2017). In addition, hundreds of thousands of mango trees are grown in home landscapes throughout central and south Florida (J. H. Crane, personal communication). Bacterial black spot (BBS), also known as bacterial canker, is caused by Xanthomonas axonopodis pv. mangiferaeindicae, most likely originating in India and spreading to other countries through the movement of contaminated plant material (Midha et al. 2012). BBS is a serious mango disease that may result in a 50% to 80% reduction in fruit production and therefore could become a limiting factor for the commercial production of some mango cultivars in Florida (Dayakar and Gnanamanickam 1995, Gagnevin and Pruvost 2001, Ploetz 2003). Moreover, even low disease severity (few lesions) on fruit can significantly reduce fruit quality and marketability. Many commercial mango cultivars are susceptible, but some are less affected by the disease than others (Cooke et al. 2009). There is little information available about how BBS affects mango cultivars in Florida and what the impact of this disease to the industry as a whole could be.
BBS has been reported in several countries in the Eastern Hemisphere, including major mango-producing areas such as Australia, Burkina Faso, China, India, Japan, Pakistan, the Philippines, South Africa, Taiwan, Thailand, and the Middle East (Gagnevin and Provost 2001, Wang et al. 2013, Jackson 2017). BBS was recorded in Hawaii in 2012 (Yasuhara-Bell et al. 2013) and was first detected in the continental United States (Palm Beach County, Florida) in 2015 (Sanajuha et al. 2016).
Symptoms
The causal agent of BBS (Xanthomonas axonopodis pv. mangiferaeindicae) infects stems, leaves, and fruit tissue through natural openings and wounds (Gagnevin and Pruvost 2001). Leaf lesions appear as raised black angular spots, frequently developing greasy margins following leaf venation, and often surrounded by a chlorotic (yellow) halo. Lesions may coalesce into large necrotic (dead) patches, and bacteria may ooze from the lesions during high-humidity conditions (Gagnevin and Pruvost 2001, Ploetz 2003). After several months of infection, leaf lesions dry and become light brown to ash-gray in color. Defoliation occurs in severe cases.
Fruit susceptibility increases as fruit matures, correlated with the weakening of the lenticels (microscopic natural openings in stems and fruits), which allows for pathogen colonization (Gagnevin and Pruvost 2001). Symptoms on fruits begin as small irregular water-soaked specks around lenticels or as small star-shaped lesions (Figure 1A–D). As the disease progresses, these lesions blacken, develop greasy margins that become raised and coalesce, and then develop cracks (Figure 1A–D). Note that these necrotic spots are angular and raised, not round and depressed into the pulp like anthracnose lesions (Figure 2). Oozing of gummy exudates (bacterial oozing) may be observed during periods of high humidity. Like anthracnose, a "tear stain" infection pattern may also occur (Figure 1E–H; Fig. 2). BBS infection may occur on immature (Figure 1E, 1G, and 1H) and mature fruit (Figure 1F). Severe fruit infections result in fruit drop. Symptoms on stems include longitudinal dark-colored cracks, which make these stems susceptible to wind breakage. Flowers and fruit peduncles can also be affected by the disease (Cooke et al. 2009).
BBS symptoms may be confused with those caused by the common anthracnose fungus, Colletotrichum gloeosporioides, a pathogen that is widespread and ubiquitous in Florida mango groves. However, there are some key differences. BBS lesions are generally confined to the peel and upper pulp, are darker and more angular, and have raised margins. Additionally, when BBS lesions coalesce, they form cracks from which sap oozes out (Figure 1F and 1G). In contrast, anthracnose lesions are dark brown to black, larger than initial BBS lesions, often semicircular in shape, and flat or sunken (not raised) (Figure 2A and 2B); eventually anthracnose lesions penetrate into the fruit pulp. Anthracnose and BBS can coinfect the same host tissue (same fruit, same leaf). When this happens, the results are large deep-rot lesions on fruits and large necrotic areas on leaves.
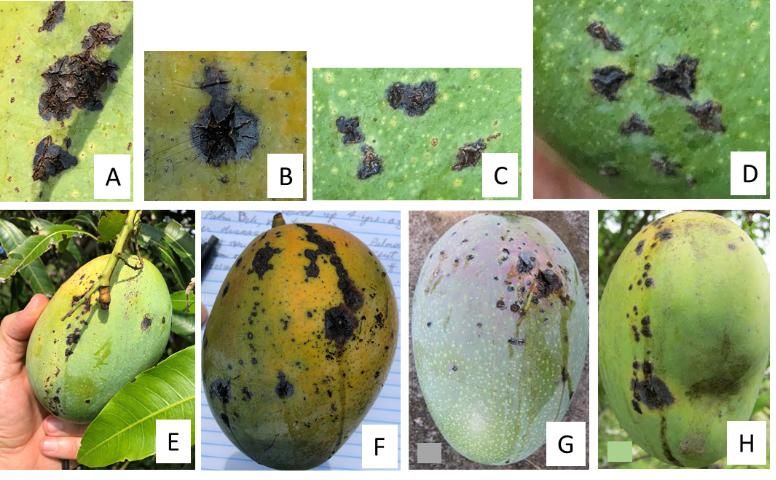
Credit: J. Crane (A, B, E, F, G, and H) and G. Sanahuja (C, D), UF/IFAS, TREC

Credit: I. Maguire, UF/IFAS, TREC
Disease Spread
Bacteria oozing from diseased tissue are dispersed by wind-driven rain (>10 mph), water splash, insects, and mechanical transmission (contaminated equipment, tools, etc.). Because the pathogen can latently infect host tissue (asymptomatic infection), infected propagation material (e.g., seedlings and grafted trees, budwood, and surface-contaminated seeds) as well as the propagation equipment and tools that come into contact with BBS (mechanical transmission) can also spread the disease (Cooke et al. 2009, Gagnevin and Pruvost 2001). Therefore, mango plants and budwood should only be sourced from reputable nurseries and healthy-looking plants.
Infection occurs through stomata of old leaves (young leaves don't have functional stomata), fruit and stem lenticels, and wounds present on leaves, stems, and fruit. Infected leaves, stems, and fruit serve as sources of inoculum, promoting a continued spread and infection of healthy new growth (Pruvost et al. 1999). High BBS incidence on fruit has been correlated with high BBS severity on leaves during the six months prior to fruit maturity (Manicom 1986). Fruit susceptibility increases with maturation (Pruvost and Luisetti 1991). High relative humidity (>80%), moderately warm temperatures (~75°F–86°F), and rainfall favor disease development (Manicom 1986, Pruvost and Luisetti 1991). BBS is particularly severe in exposed, windy areas where adequate windbreaks are not present. This is because windblown soil particles and debris as well as stem and fruit scars caused by nearby stems during windy weather result in microscopic peel damage where the BBS pathogen can enter. The pathogen does not survive on the ground in soil or in dead leaves (Provust and Manicom 1993). Disease incidence declines rapidly during dry periods.
Resistant cultivars have not yet been developed, and there is limited information on disease susceptibility and tolerance of the many mango cultivars currently in Florida and other areas (Gagnevin and Pruvost 2001). The information on response of mango cultivars to BBS is tentative and based only on local observations and published scientific literature (Table 1). This list will likely change as new data are generated. "Highly susceptible" implies severe and relatively fast BBS symptom development on fruit. Commercial fruit production under high BBS pressure conditions may be very difficult for cultivars under this category. "Moderately susceptible" and "less susceptible" imply significantly less BBS symptom development on fruit, especially when an integrated BBS management plan is implemented.
Management
Use an integrated management strategy to minimize BBS incidence in the grove. This includes management strategies previously published (Gagnevin and Pruvost 2001, Jackson 2017):
- Establish plantings in areas with a dry climate. This is not possible in Florida because the rainy season generally extends from May through September and the dry season from October through April. However, in Florida, up to about one-third of the annual rainfall occurs during the dry season.
- Use disease-free rootstocks and scion-wood when propagating new plants. Before introducing nursery plants into your grove, inspect them for signs of BBS—if present, do not purchase the tree, and replace it with a nonsymptomatic tree. Spray all new trees to be introduced to the grove with bactericide (copper-based products) prior to bringing them into the grove.
- Grow cultivars with less or moderate susceptibility to BBS. Cultivars with moderate susceptibility to BBS may be able to be grown with a good integrated management strategy.
- Grow early-season cultivars to avoid prolonged exposure of fruits to high precipitation—that is, avoid the rainy season as much as possible.
- Establish windbreaks to reduce pathogen spread by wind and microdamage to leaves, stems, and fruit that can create openings for the pathogen.
- Avoid establishing an irrigation system that wets the canopy (e.g., a high-volume overhead system); instead, install low-volume irrigation systems that do not wet the aboveground parts of the tree (e.g., drip or microsprinkler).
- Apply copper-based bactericides beginning at panicle emergence through the harvest season to suppress BBS pathogen populations. Applications during the rainy season are especially important. Apply copper-based bactericides at a two-to-four-week interval (according to label instructions).
- Prune trees annually to limit tree height and to maintain an open canopy. An open canopy improves air movement and light penetration, which will reduce the hours of leaf, fruit, and stem wetness due to morning dew, rainfall, and overhead irrigation.
- Sanitize pruning tools and equipment between trees. Remove and destroy infected plant parts.
- Harvest fruit by clipping fruit stalks, rather than tearing fruit from their stems, to avoid causing open wounds to stems.
Windbreaks
Establishment of windbreaks to reduce wind speeds and microdamage to plant parts is recommended. However, establishment of windbreaks takes time and reduces land area planted with mango trees. For windbreaks, select plant species that are noninvasive and that establish quickly. Space the windbreak at the tree row spacing or wider to allow unimpeded machine traffic. Periodic pruning to limit the height of the windbreak is important; otherwise excessive shading to the adjacent mango trees may occur. For more information, visit UF/IFAS EDIS (https://edis.ifas.ufl.edu) and Andreu et al. (2017), The Benefits of Windbreaks for Florida Growers (https://edis.ifas.ufl.edu/fr253).
Bactericides
Periodic applications of copper-based bactericides may reduce the incidence of BBS, although fruit from highly BBS-susceptible cultivars may still be affected. Fungicides will not control BBS. Please contact your local county Extension agent for recommendations. For a listing of disease control products, visit the Crop Data Management Systems, Inc. website (http://www.cdms.net/) (no endorsement intended).
Special Acknowledgement
The authors would like to sincerely thank Mr. Alex Salazar, Tropical Acres Farms, West Palm Beach, Florida and Mr. Gary Zill, Zill High Performance Plants, Lake Worth, Florida, for their invaluable input and review of this factsheet. Their expertise is greatly appreciated.
Literature Cited
Ah-You, N., L. Gagnevin, P. A. D. Grimont, S. Brisse, X. Nesme, F. Chiroliu, L. Bui Thi Ngoc, E. Jouen, P. Lefeuvre, C. Vernére, and O. Pruvost. 2009. "Polyphasic Characterization of Xanthomonads Pathogenic to Members of the Anacardiaceae and Their Relatedness to Species of Xanthomonas." International J. Systemic and Evolutionary Microbiology 59:306–318.
Coates, L., C. Akem, T. Cooke, E. Dann, and A. Young. 2009. In Diseases of Fruit Crops in Australia, edited by T. Cooke, D. Persley, and S. House. 157–174. Collingwood, VIC, Australia: CSIRO Publishing.
Crane, J. H. 2017. "Tropical Fruit Production in Florida—Trials, Tribulations, and Opportunities." Proc. Fla. State Hort. Soc. 131:ix–xii.
Crane, J. and R. Gazis. 2020. Bacterial black spot (BBS) of mango in Florida. UF/IFAS Extension https://edis.ifas.ufl.edu/publication/HS1369
Dayakar, B. V., and S. S. Gnanamanickam. 1995. "Xanthomonas campestris pv. mangiferaeindicae from Southern India." Indian Phytopathology 49 (3): 227–233.
Gagnevin, L., and O. Pruvost. 2001. "Epidemiology and Control of Mango Bacterial Black Spot." Plant Disease 85 (9): 928–935.
Jackson, G. 2017. "Mango Bacterial Black Spot (213)." Pacific Pests and Pathogens – Fact Sheets (App). Australian Centre for International Agricultural Research.
Manicom, B. Q. 1986. "Factors Affecting Bacterial Spot of Mangos Caused by Xanthomonas campestris pv. mangiferaeindicae." Ann. Applied Biology 109:129–135.
Midha, S., M. Ranjan, V. Sharma, A. K. Pinnaka, and P. B. Patil. 2012. "Genome Sequence of Xanthomonas citri pv. mangiferaeindicae Strain LMG 941." Journal of Bacteriology 194 (11): 3031.
Ploetz, R. C. 2003. "Diseases of Mango." In Diseases of Tropical Fruit Crops, edited by R. C. Ploetz. 332–334. Wallingford, Oxon, UK: CABI International.
Pruvost, O., C. Boyer, P. Grygiel, K. Boyer, C. Veriere, L. Gagnevin, S. Soro, C. N'Guessan, and D. Kone. 2014. "First Report of Xanthomonas citri pv. mangiferaeindicae Causing Mango Bacterial Canker on Mangifera indica in Ivory Coast." Disease Notes 98 (12): 1740. https://doi.org/10.1094/PDIS-07-14-0669-PDN.
Pruvost, O., C. Boyer, K. Vital, C. Verniere, L. Gagnevin, L. de Bruno Austin, and J. Y. Rey. 2011. "First Report in Ghana of Xanthomonas citri pv. mangiferaeindicae Causing Mango Bacterial Canker on Mangifera indica L." Disease Notes 95 (6): 774. https://doi.org/10.1094/PDIS-02-11-0098
Pruvost, O., C. Boyer, K. Vital, C. Verniere, L. Gagnevin, and I. Somda. 2011. "First Report in Burkina Faso of Xanthomonas citri pv. mangiferaeindicae Causing Mango Bacterial Canker on Mangifera indica." Disease Notes 95 (10): 1312. https://doi.org/10.1094/PDIS-04-11-0324
Pruvost, O., C. Boyer, K. Vital, C. Verniere, L. Gagnevin, and Y. N. Traore. 2012. "First Report in Mali of Xanthomonas citri pv. mangiferaeindicae Causing Mango Bacterial Canker on Mangifera indica." Disease Notes 96 (4): 581. https://doi.org/10.1094/PDIS-01-12-0001-PDN.
Pruvost, O., A. Couteau, and J. Luisetti. 1999. "Development of Bacterial Black Spot of Mangoes and Epiphytic Populations of the Pathogen (Xanthomonas campestris pv. mangiferaeindicae) under Natural Conditions." Fruits 45 (2): 125–140.
Pruvost, O., A. Couteau, X. Perrier and J. Luisetti. 1998. "Phenotypic Diversity of Xanthomonas sp. mangifereaeindicae." J. Applied Microbiology 84:115–124.
Pruvost, O., and J. Luisetti. 1991. "Effect of Time of Inoculation with Xanthomonas campestris pv. mangiferaeindicae on Mango Fruits Susceptibility Epiphytic Survival of X. c. pv. mangiferaeindicae on Mango Fruits in Relation to Disease Development." J. Phytopathology 133 (2): 139–151.
Pruvost, O., and B. Q. Manicom. 1993. "Xanthomonas campestris pv. mangiferaeindicae: Cause of Bacterial Black Spot of Mangos." In Xanthomonas, edited by J. G. Swings and E. L. Civerolo. 91–95. London: Chapman and Hall.
Sanahuja, G., R. C. Ploetz, P. Lopez, J. L. Konkol, and A. J. Palmateer. 2016. "Bacterial Black Spot of Mango, Mangifera indica, Caused by Xanthomonas citri pv. mangiferaeindicae, Confirmed for the First Time in the Western Hemisphere." Poster presented at the American Phytopathological Society Annual Meeting, Tampa, FL, July 30–Aug. 3, 2016.
Sanahuja, G., R. C. Ploetz, P. Lopez, J. L. Konkol, A. J. Palmateer, and O. Pruvost. 2016. "Bacterial Canker of Mango, Mangifera indica, Caused by Xanthomonas citri pv. mangiferaeindicae, Confirmed for the First Time in the Americas." Disease Notes 100 (12): 2520. https://doi.org/10.1094/PDIS-03-16-0412-PDN
Wang, S., W. Wu, W. Ma, Z. Ma, R. Zhan, Q. Yao, G. Sun, and J. Xie. 2013. "Evaluation of Sixteen Introduced Mango Cultivars in Zhangjiang, China." Acta Hort. 992:211–220.
Yasuhara-Bell, J., A. S. de Silva, A. M. Alvarez, R. Shimabuku, and M. Ko. 2013. "First Report in Hawai'i of Xanthomonas citri pv. mangiferaeindicae Causing Bacterial Black Spot on Mangifera indica." Plant Disease 97 (9): 1244. https://doi.org/10.1094/PDIS-11-12-1071-PDN
Zombré, C., P. Sankara, S. L. Ouédraogo, I. Wonni, O. Pruvost, C. Boyer, C. Verniére, A. Adandonon, J. F. Vayssiéres, and C. Ahohuendo. 2015. "First Report of Xanthomonas citri pv. mangiferaeindicae Causing Mango Bacterial Canker on Mangifera indica L. in Benin." Disease Notes 99 (12): 1854. https://doi.org/10.1094/PDIS-04-15-0392-PDN
Zombré, C., I. Wonni, S. L. Ouédraogo, K. E. Kpemoua, K. Assignon, P. Sankara, C. Verniére, C. Boyer, K. Boyer, S. Javegny, and O. Pruvost. 2016. "First Report of Xanthomonas citri pv. mangiferaeindicae Causing Mango Bacterial Canker on Mangifera indica L. in Togo." Disease Notes 101 (3): 503. https://doi.org/10.1094/PDIS-09-16-1259-PDN