Introduction
The lone star tick, Amblyomma americanum, was first described by Linnaeus in 1758. Lone star ticks feed on the blood of various animals (domesticated and wild) as well as humans. The tick was first considered a nuisance as it does not transmit the etiological agent of Lyme disease, but more recent studies have shown that this species can transmit various other pathogens to humans and other animals, such as those that cause ehrlichiosis, rickettsiosis, tularemia, and theileriosis.

Credit: Lyle Buss, UF/IFAS
Synonymy
Acarus americanus Linnaeus, 1758
Acarus nigua De Geer, 1778
Rhynchoprion americanum Hermann, 1804
Ixodes nigua Latreille, 1804
Ixodes americanus Fabricius, 1805
Ixodes orbiculatus Say, 1821
Euthesius americanus Gistel, 1848
Ixodes unipunctata Packard, 1869
Ixodes unipictus Verrill, 1870
Amblyomma unipunctum Packard, 1870
Ixodes nigra Cobbold, 1879
Amblyomma foreli Stoll, 1890
Amblyomma unipunctatum Thurow, 1891
Ixodes unipuncta Lewis, 1899
Ixodes orbicularis Neumann, 1901
Amblyomma (Anastosiella) americanum Santos Dias, 1993
Amblyomma (Amblyomma) americanum Camicas et al., 1998
From the Catalogue of Life: 2009 Annual Checklist (ITIS 2013)
Distribution
The lone star tick is widely distributed across the eastern, southeastern and midwestern US (Figure 2) (Childs and Paddock 2003). However, the tick may establish local populations outside of this range (Childs and Paddock 2003). The tick reportedly has been expanding its range north and west out of the historic range depicted in the distribution map provided by the CDC (Figure 2) (Childs and Paddock 2003). The lone star tick typically is found in second growth woodland habitats that have populations of white-tailed deer (Odocoileus virginianus) (Kollars 1993).
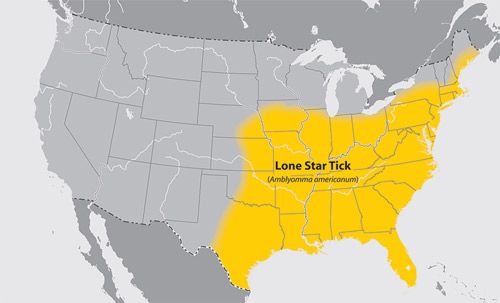
Credit: Centers for Disease Control and Prevention
With the re-introduction and increased populations of white-tailed deer in many areas of the eastern US, the ticks may further expand their range through transportation while feeding on white-tailed deer, a key host (Paddock and Yabsley 2007). Wild turkey populations also are a common host and may contribute to tick expansion by providing additional hosts for immature stages (Kollars et al. 2000). In some Midwestern states the lone star tick is colloquially known as the "turkey tick" due to its association with wild turkeys (Childs and Paddock 2003).
Description and Life Cycle
Adult lone star ticks are brown with eight legs and long mouthparts (Figure 1). Lone star ticks are similar in body size when compared to other ticks like the American dog tick Dermacentor variabilis (Say), and the brown dog tick, Rhipicephalus sanguineus Latreille, but are larger than the blacklegged tick or deer tick, Ixodes scapularis Say. Adult lone star ticks exhibit sexual dimorphism: the female has a silvery-white spot near the center of her back on the posterior portion of the shield (scutum) and the male has varied white streaks or spots around the margins of the top of its body (Drees and Jackman 1999).
Lone star ticks are three-host ticks, feeding on different hosts during the larval, nymphal, and adult stages. The ticks have piercing-sucking mouthparts with chelicerae that pierce through the skin of the host. Attachment is facilitated by the tubular hypostome and a secreted cement- or latex-like compound that attaches ("glues") the tick to the host until feeding is complete (Adams et al. 2003). After feeding once in each larval, nymphal, and adult stage, the tick withdraws the mouthparts and drops to the ground to molt or oviposit, as described below.
A tick lifecycle begins when the blood-engorged female tick falls from the host and after several days deposits ~5,000 eggs on the soil in a "protected" location, such as in mulch or leaf litter (NCIPMI 1998). After dislodging from the host, the female will seek a microclimate, typically an area of high humidity at a soil level that is best suited for survival of the eggs (Patrick and Hair 1979). Females have been shown to search for a favorable microclimate up to 61 cm from where they were experimentally placed on the ground after feeding (Patrick and Hair 1979). Following an incubation period, larvae hatch from eggs and progress through a quiescent (resting) period, then seek a host by questing.
Questing is a behavior that entails climbing up an object, like a blade of grass, and waiting for a host to touch the larva. The larva then grasps the host and proceeds to move about the host, seeking a preferred feeding site. After acquiring a host, the larva attaches, blood-feeds for 1–3 days, detaches its mouthparts, and then drops from the host to digest its blood meal and molt into a nymph. Nymphs repeat this process; however, after dislodging from this second host they molt into adults. Sizes of ticks in each stage can vary due to genetic and environmental conditions (Koch 1986). In laboratory settings, the life cycle can be shortened to less than 22 weeks under optimal conditions, but is usually 2 years in nature (Troughton and Levin 2007).
Seasonal peaks in the population have been reported for lone star ticks in Georgia; adult numbers peak April to June, nymphs had a bimodal distribution during May to July and August to September, whereas numbers of larvae peak July to September (Semtner and Hair 1973). Seasonality in Missouri was similar, wherein peak activity of adults was between May and July, nymphs in May to August, and larvae in July through September (Kollars et al. 2000). Anecdotal reports in Florida suggest that one of the three active stages of lone star ticks can be active in nearly every month of the year; however, peaks in activity likely are similar to those observed in Georgia.

Credit: Chris Holderman, UF/IFAS
Eggs
Eggs (Figure 4) are glossy, brown, oval structures that are approximately 0.4 mm in width by 0.5 mm in length (NCIPMI 1998). Eggs are deposited by engorged females in the spring, summer, and autumn. Survival rates were highest in the spring and autumn egg clutches. Incubation time in a field study was temperature dependent, ranging from 31 to 60 or more days. However, desiccation of the eggs readily occurred when soil moisture was low (< 3%) and soil surface temperature was greater than 40°C or 104°F (Patrick and Hair 1979). Because a female lays all of her eggs at one time, they are typically found in a large mass.

Credit: Lyle Buss, UF/IFAS
Larvae
Larvae, typically called "seed ticks" due to their small size and abundance (Figure 5), are 0.5 to 1.0 mm long and have six legs (NCIPMI 1998). If humidity and temperature are favorable the larvae can survive for up to six months in the environment, but typically the larval stage is shorter due to acquisition of a suitable host (Troughton and Levin 2007). After feeding on the host for 4 to 9 days, the larva drops off and, in 3 to 4 weeks, molts into the nymphal stage (Troughton and Levin 2007). When larvae are encountered before host location, several thousand of them can be in a small area.

Credit: Chris Holderman
Nymphs
Nymphs (Figure 6) are 1.5 to 2.5 mm in length and have eight legs (NCIPMI 1998). Nymphs can survive for up to six months without feeding on a host. Once a host is located they feed for 3 to 8 days, drop off the host and molt into the adult stage within a 5 to 6 week period (Troughton and Levin 2007).

Credit: Chris Holderman
Adults
Adults (Figures 7 and 8) are 3 to 4 mm in length and have eight legs. Adults can survive 8 months to 2 years without feeding if temperature and humidity are favorable (Troughton and Levin 2007). Mating occurs on the host. The male must feed to produce spermatophores, and the female must feed to produce eggs (Troughton and Levin 2007). Blood meals increase female size drastically (Figures 4 and 9). While feeding, the female emits pheromones that stimulate the male to detach, locate the female, and mate with her (Sonenshine 2004). Males may mate with multiple females before dying. Once the female mates, she blood-feeds for several days, reaching an engorged state and then leaves the host to find a location to lay her eggs. After laying her eggs, the female dies (NCIPMI 1998).

Credit: Lyle Buss, UF/IFAS

Credit: Lyle Buss, UF/IFAS

Credit: Lyle Buss, UF/IFAS
Hosts
Although ticks are mobile, hosts are the primary means of tick dispersal for all active life stages (Barnard et al. 1988). The lone star tick is very aggressive and non-specific when seeking hosts (Goddard and Varela-Stokes 2009), although some specificity does occur within each life stage. The lone star tick can be found on humans, domesticated animals (e.g. cattle, dogs, horses, goats), ground-dwelling birds (e.g., quail and wild turkeys), and small (e.g. squirrels, opossums, hares) and large (primarily white-tailed deer and coyotes) wild mammals (Cooley and Kohls 1944, Bishopp and Trembley 1945, Kollars et al. 2000). Larvae primarily are collected from birds and mammals, but not on small rodents, while nymphs feed on all of these animals (Barnard et al. 1988, Kollars, et al. 2000). Adults typically feed on large- or medium-sized mammals, but can be found on small rodents and wild turkeys. With the exception of wild turkeys, adult lone star ticks infrequently feed on birds (Barnard et al. 1988, Kollars et al. 2000, Mock et al. 2001).
Medical and Veterinary Importance
The lone star tick is the most common tick reported to bite humans in the southeastern and southcentral US (Masters et al. 2008). Various pathogens have been shown to enter the tick by blood feeding; however, most are not transmitted because the tick is not a competent vector (Goddard and Varela-Stokes 2009). An unknown pathogen has been implicated in causing Southern tick-associated rash illness (STARI) in humans, but etiology, pathogenicity and identification are pending (CDC 2011a). Several pathogens are known to be transmitted by the lone star tick and given the proper circumstances they may manifest into disease. These include ehrlichiosis, rickettsiosis, tularemia and protozoan infections. The causative agents of ehrlichiosis, rickettsiosis, and anaplasmosis are all tick-borne bacterial infections that are readily treatable in humans, but the causative agent can be difficult to determine because the similarities among the pathogens.
Recently, in three case studies, including one specifically identified as a result of a bite by a lone star tick, tick bites may have been involved in producing or generating an immune response that caused a food allergy to red meat proteins (Wolver et al. 2009). Heavy infestations of lone star ticks also have been associated with increased mortality in white-tailed deer fawns in Oklahoma (Bolte et al. 1970).
Southern Tick-Associated Rash Illness—STARI
STARI, also known as Masters disease, is a medical condition with a currently unknown etiology, the pathogen of which is suspected by some scientists to be transmitted by the lone star tick (CDC 2011a, Masters et al. 2008). STARI was first thought to be Lyme disease, caused by Borrelia burgdorferi infections, but this hypothesis has been dismissed due to the inability of the lone star tick to transmit the Borrelia burgdorferi spirochete, as well as the bacteria being digested within the tick (Mukolwe et al. 1992).
A spirochete was isolated from a STARI patient and named Borrelia lonestari; however, the next two-dozen STARI patients were not infected with Borrelia lonestari (CDC 2011a). The symptoms usually manifest within seven days after a lone star tick bite, with a rash expanding three inches or more from the bite location (CDC 2011a). STARI patients usually exhibit fatigue, fever, headache, and joint and muscle pain, but symptoms have been resolved following antibiotic treatments (CDC 2011a). STARI is usually diagnosed within the southern U.S.A. but has been reported in one case as far north as New York state (Feder et al. 2011). The cause of STARI is currently unknown (CDC 2011a).
Ehrlichiosis
Ehrlichiosis is a disease caused by a group of obligate intracellular pathogenic bacteria, meaning they reside within the host animal's cells. Both humans and other animals can be affected. Ehrlichia chaffeensis is the cause of human monocytic ehrlichiosis (HME), of which the lone star tick is the primary vector to humans (CDC 2011b). White-tailed deer are thought to be an important reservoir host for Ehrlichia chaffeensis, while rabbits and squirrels may play a role in maintaining the pathogen in the U.S.A. (Allan et al. 2010a). Diagnosis of Ehrlichiosis outside of the lone star tick's range often is attributed to a misdiagnosis of anaplasmosis, a disease caused by a different pathogen (CDC 2011b). Confirmed human ehrlichiosis cases have increased annually from 142 in 2001 to 885 in 2012 (CDC 2012b).
The lone star tick transmits Ehrlichia ewingii, but the prevalence of this pathogen is much lower, with only 10 human cases reported in 2010 (CDC 2012b). Ehrlichia ewingii is likely maintained in nature by lone star ticks feeding upon ruminants, squirrels, and hares (Allan et al. 2010a).
A species of Ehrlichia, not yet identified, and a potential new zoonosis (animal-human disease), produces Panola mountain Ehrlichia (PME). This pathogen, isolated near Atlanta, GA, shows genetic similarity to Cowdria ruminatium, the causative agent of the devastating disease heartwater in ruminants such as cattle (Loftis et al. 2006).
Dogs have been shown to be susceptible to infections of both Ehrlichia chaffeensis and Ehrlichia ewingii. However, dogs also are susceptible to Ehrlichia canis a related microbe, which is usually transmitted by Rhipicephalus sanguineus (Latrielle), the brown dog tick (Beall et al. 2012).
Rickettsiosis
Rickettsiosis is a disease caused by infection of one of several bacteria in the genus Rickettsia. These bacteria are obligate intracellular pathogens as described with ehrlichiosis. A Rickettsia species, similar to Rickettsia rickettsii, which causes Rocky Mountain spotted fever (RMSF) in humans, has been isolated from lone star ticks in a laboratory setting by Goddard and Norment (1986). Rickettsia rickettsii has not been found in lone star ticks. Rickettsia parkeri has been confirmed in laboratory transmission studies to be vectored by lone star ticks (Goddard 2003) and has been isolated from a small number of field-collected lone star ticks (Cohen et al. 2009).
Traditionally, Rickettsia parkeri was thought to be non-pathogenic (non-disease causing) in humans, but Raoult and Olson (1999) expressed the opinion that all rickettsial organisms should be viewed as potential human pathogens. Due to limitations in diagnosis of the specific microbe species, the CDC does not distinguish among individual bacterial pathogen species. For 2012, 3,776 cases were attributed to "probable" and 179 to "confirmed" cases of human infections of spotted fever "group" rickettsiosis in the US (CDC 2013).
Tularemia
Tularemia is a disease caused by a bacterium (Francisella tularensis) that affects many mammals, including humans, and is spread primarily by infected arthropods, including ticks, or by contact with infected mammals, usually rabbits and hares (Hopla 1960). Recently, tularemia incidence has ranged between 93 and 166 cases (2007–2011), but these are not segregated by infection type or route. However, most infections are associated with hunters handling infected rabbits (CDC 2012b).
Theileria cervi
Theileria cervi is a tick-transmitted protozoan parasite that infects white-tailed deer and attacks red blood cells. This pathogen has been linked with deer death when tick numbers and parasite numbers are substantial (Samuel and Trainer 1970). The role of Theileria cervi in deer mortality is somewhat unclear, however, given that some researchers have reported fawn mortality in deer populations infested with lone star ticks without the presence of Theileria cervi (Hair et al. 1992). The incidence rate of Theileria cervi increased in white-tailed deer populations from June to September, which corresponded to lone star tick activity in the area surveyed (Waldrup et al. 1992).
Management
Many management practices have been evaluated for the reduction of tick numbers in a local area. Typical practices are acaricide (insecticides for ticks) applications, vegetative management (controlled burning or mechanical removal of under-story brush and other plants), and host exclusion. Many involve greatly altering the biotic and abiotic factors that contribute to tick population increases.
Controlled burning over a four-year study in a wooded recreational area resulted in a significant reduction in lone star tick populations (Davidson et al. 1994). Because the tick lifecycle is 2 to 3 years in nature, tick reduction following such a management protocol may not be apparent for some time.
Treatment of deer with a "4-poster" feeder station has reduced tick numbers over time (Solberg et al. 2003). The "4-poster" feeder station attracts deer with corn. While eating the corn their ears, head and neck come in contact with acaricide-treated paint rollers. Over a three-year study, ticks (in this case, Ixodes scapularis) were reduced on deer (adult tick hosts) and mice (larval and nymphal tick hosts) (Solberg et al. 2003). Because lone star ticks also use deer as hosts, such an approach may work where these ticks are found. A Tennessee study evaluated this feeder station system; however, although tick populations were reduced, the costs of such a station were high (est. $20 per device per week) and the impact was limited in range (one station per 20 hectares, ~50 acres) (Harmon 2010).
In a study examining individual actions and combinations of acaricide application, vegetative management, and host exclusion (white-tailed deer), an integrated pest management system using all three approaches was reported to be the most effective. However, this approach was dependent on resources available (equipment, supplies and personnel costs) to implement the management practices (Bloemer et al. 1990). Altering host (white-tailed deer) behavior by removing an invasive shrub was shown to alter deer habitat preference and therefore tick prevalence in the habitat (Allan et al. 2010b).
The use of a repellent or pesticide, correctly applied to clothing and on gear following specific product label instructions, is considered the best tick bite prevention as recommended by the CDC (2012a). Wearing light colored clothing, inspecting clothing, gear, and pets, conducting a full body tick check, and showering after being outdoors are all recommended steps toward preventing tick bites. For protection of cats and dogs against tick bites, please refer to the EDIS publications below and consult with your veterinarian.
For more information see the UF/IFAS Insect Management Guide for ticks.
Selected References
Adams DR, Anderson BE, Ammirati CT, Helm KF. 2003. Identification and diseases of common U.S. ticks. The Internet Journal of Dermatology 2:1. DOI: 10.5580/1189.
Allan BF, Goessling LS, Storch GA, Thach RE. 2010a. Blood meal analysis to identify reservoir hosts for Amblyomma americanum ticks. Emerging Infectious Diseases 16: 433–440.
Allan BF, Dutra HP, Goessling LS, Barnett K, Chase JM, Marquis RJ, Pang G, Storch GA, Thach RE, Orrock JL. 2010b. Invasive honeysuckle eradication reduces tick-borne disease risk by altering host dynamics. PNAS 107: 18523–18527.
Barnard DR, Mount GA, Koch HG, Haile DG, Garris GI. 1988. Management of the lone star tick in recreation areas. U.S. Department of Agriculture Handbook 682. 33pp. Agriculture Research Service.
Beall MJ, Alleman AR, Breitschwerdt EB, Cohn LA, Couto CG, Dryden MW, Guptill LC, Lazbik C, Kania SA, Lathan P, Little SE, Roy A, Sayler KA, Stillman BA, Welles EG, Wolfson W, Yabsley MJ. 2012. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in dogs in North America. Parasites and Vectors 5:29. DOI:10.1186/1756-3305-5-29.
Bishopp FC, Trembley HL. 1945. Distribution and hosts of certain North American ticks. Journal of Parasitology 31: 1–54.
Bloemer SR, Mount GA, Morris TA, Zimmerman RH, Barnard DR, Snoddy EL. 1990. Management of lone star ticks (Acari: Ixodidae) in recreational areas with acaricide applications, vegetative management, and exclusion of white-tailed deer. Journal of Medical Entomology 27: 543–550.
Bolte JR, Hair, JA, Fletcher J. 1970. White-tailed deer mortality following tissue destruction induced by lone star ticks. The Journal of Wildlife Management 34: 546–552.
CDC. (2011a). Centers for Disease Control and Prevention. Southern Tick-Associated Rash Illness. (10 June 2013).
CDC. (2011b). Centers for Disease Control and Prevention. Ehrlichiosis. (10 June 2013).
CDC. (2012a). Centers for Disease Control and Prevention. Avoiding Ticks. (10 June 2013).
CDC. 2012b. Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report, Summary of Notifiable Diseases - United States, 2010. 59: 1–116.
CDC. 2013. Centers for Disease Control and Prevention. Notifiable Diseases and Mortality Tables, Morbidity and Mortality Weekly Report (MMWR). 61: ND-719-ND-732.
Childs JE, Paddock CD. 2003. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annual Review of Entomology 48: 307–337.
Cohen SB, Yabsley MJ, Garrison LE, Freye JD, Dunlap BG, Dunn JR, Mead DG, Jones TF, Moncayo AC. 2009. Rickettsia parkeri in Amblyomma americanum ticks, Tennessee and Georgia, USA. Emerging Infectious Diseases 15: 1471–1473.
Cooley RA, Kohls GM. 1944. The genus Amblyomma (Ixodidae) in the United States. Journal of Parasitology 30: 77–111.
Davidson WR, Siefken DA, Creekmore LH. 1994. Influence of annual and biennial prescribed burning during March on the abundance of Amblyomma americanum (Acari: Ixodidae) in central Georgia. Journal of Medical Entomology 31: 72–81.
Drees BM, Jackman J. (1999). A Field Guide to Common Texas Insects. Lone star tick. (07 February 2022).
Feder HM, Hoss DM, Zemel L, Telford III SR, Dias F, Wormser GP. 2011. Southern tick associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clinical Infectious Diseases 53:e142-e146. DOI: 10.1093/cid/cir553.
Goddard J, Norment BR. 1986. Spotted fever group Rickettsiae in the lone star tick, Amblyomma americanum (Acari: Ixodidae). Journal of Medical Entomology 23: 465–472.
Goddard J. 2003. Experimental infection of lone star ticks, Amblyomma americanum (L.), with Rickettsia parkeri and exposure of guinea pigs to the agent. Journal of Medical Entomology 40: 686–689.
Goddard J, Varela-Stokes AS. 2009. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Veterinary Parasitology 160: 1–12.
Hair JA, Hoch AL, Buckner RG, Barker RW. 1992. Fawn hematology and survival following tick infestation and theileriasis. Journal of Agricultural Entomology 9: 301–319.
Harmon JR. 2010. Management of ticks and tick-borne disease in a Tennessee retirement community. Thesis, University of Tennessee, Knoxville, TN.
Hopla CE. 1960. The transmission of tularemia organisms by ticks in the southern states. Southern Medical Journal 53: 1–6.
ITIS (2013). Integrated Taxonomic Information System. Amblyomma americanum (Linnaeus, 1758). (03 October 2013).
Koch HG. 1986. Development of the lone star tick, Amblyomma americanum (Acari: Ixodidae), from immatures of different engorgement weights. Journal of the Kansas Entomological Society 59: 309–313.
Kollars TM. 1993. Ticks (Acari: Ixodidae) infesting medium-sized wild mammals in southwestern Tennessee. Journal of Medical Entomology 30: 896–900.
Kollars Jr, TM, Oliver Jr, JH, Durden LA, Kollars PG. 2000. Host associations and seasonal activity of Amblyomma americanum (Acari: Ixodidae) in Missouri. The Journal of Parasitology 86: 1156–1159.
Loftis AD, Reeves WK, Spurlock JP, Mahan SM, Troughton DR, Dasch GA, Levin ML. 2006. Infection of a goat with a tick-transmitted Ehrlichia from Georgia, U.S.A., that is closely related to Ehrlichia ruminantium. Journal of Vector Ecology 31: 213–223.
Masters EJ, Grigery CN, Masters RW. 2008. STARI or Masters Disease: Lone star tick-vectored Lyme-like illness. Infectious Disease Clinics of North America 22: 361–376.
Mock DE, Applegate RD, Fox LB. 2001. Preliminary survey of ticks (Acari: Ixodidae) parasitizing wild turkeys (Aves: Phasianidae) in Eastern Kansas. Journal of Medical Entomology 38: 118–121.
Mukolwe SK, Kocan AA, Barker RW, Kocan KM, Murphy GL. 1992. Attempted transmission of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) (JDI strain) by Ixodes scapularis (Acari: Ixodidae), Dermacentor variabilis, and Amblyomma americanum. Journal of Medical Entomology 29: 673–677.
NCIPMI. (1998). Insect and related pests of man and animals. North Carolina Integrated Pest Management Information. (10 June 2013).
Patrick CD, Hair JA. 1979. Oviposition behavior and larval longevity of the lone star tick, Amblyomma americanum (Acarina: Ixodidae), in different habitats. Annals of the Entomological Society of America 72: 308–312.
Raoult D, Olson JG. 1999. Emerging rickettsioses, pp. 17-35 In: Scheld WM, Craig WA, and Hughes JM [eds.], Emerging infections, Vol. 3. ASM Press, Washington, DC.
Samuel WM, Trainer DO. 1970. Amblyomma (Acarina: Ixodidae) on white-tailed deer, Odocoileus virginianus (Zimmermann), from south Texas with implications for theileriasis. Journal of Medical Entomology 7: 567–574.
Semtner PJ, Hair JA. 1973. The ecology and behavior of the lone star tick (Acarina: Ixodidae). Journal of Medical Entomology 10: 618–628.
Solburg VB, Miller JA, Hadfield T, Burge R, Schech JM, Pound JM. 2003. Control of Ixodes scapularis (Acari: Ixodidae) with topical self-application of permethrin by white-tailed deer inhabiting NASA, Beltsville, Maryland. Journal of Vector Ecology 28: 117–134.
Sonenshine DE. 2004. Pheromones and other semiochemicals of ticks and their use in tick control. Parasitology 129: S405–S425.
Troughton DR, Levin, ML. 2007. Life cycles of seven ixodid tick species (Acari: Ixodidae) under standardized laboratory conditions. Journal of Medical Entomology 44: 732–740.
Waldrup KA, Moritz J, Baggett D, Magyar S, Wagner GG. 1992. Monthly incidence of Theilria cervi and seroconversion to Babesia odocoilei in white-tailed deer (Odocoileus virginanus) in Texas. Journal of Wildlife Diseases 28: 457–459.
Wolver SE, Sun DR, Commins SP, Schwartz LB. 2012. A peculiar cause of anaphylaxis: no more steak? The journey to discovery of a newly recognized allergy to galactose-alpha-1,3-galactose found in mammalian meat. Journal of General Internal Medicine 28: 322–325.