Introduction
Varroa destructor (Figure 1) is considered the most devastating pest of western honey bees (Apis mellifera L.) globally. Its natural host is A. cerana, the Asian honey bee. Apis mellifera (hereafter "honey bee") became an additional host and is very susceptible to damage caused by the mite. These mites are found wherever the honey bee occurs (Iwasaki et al. 2015). Varroa effectively vectors multiple viruses (Rosenkranz et al. 2010), which can severely weaken or cause the collapse of honey bee colonies if left untreated (Thompson et al. 2014; Frey and Rosenkranz 2014).

Credit: Scott Bauer, USDA
Varroa have two stages in their lifecycle: the phoretic and reproductive stages. Phoretic Varroa are adult females who are attached to and feed on adult bees. After a period of 1–10 days (Beetsma et al. 1999), the phoretic mites enter open honey bee brood cells (wax cells each containing a single bee larva), hide under the developing bee larva, and wait for the cell to be capped. Once the cell is capped, the adult female (now called a "foundress mite") begins to feed on the developing prepupa/pupa and starts to lay eggs, thus initiating the reproductive stage. Varroa prefer to feed on drone (male) brood because drones take longer to develop than do worker bees, affording Varroa the ability to produce more offspring on the former. That said, Varroa commonly are found in capped cells containing worker brood because the amount of drone brood in a colony generally is restricted. The Varroa eggs hatch in the capped cell and the resulting immature mites feed on the developing honey bee pupa contained within the cell and mate. Foundress mites that are still alive and mature female daughter mites emerge from the cell with the adult bee, thus initiating the phoretic stage. The male mites remain in the cell, where they die. The mated female mites continue to feed on the adult bee until they are ready to reproduce (Rosenkranz et al. 2010).
Why monitor colonies?
It is critical to control Varroa before they reach levels that threaten colony productivity and survival, rather than respond after the damage has occurred. Therefore, beekeepers should monitor Varroa populations in their colonies so they can make informed management decisions and apply the most appropriate control techniques. The main objective with sampling is to determine the percentage mite infestation, which is usually expressed as mites/100 adult bees and called infestation rate. The infestation rate that is harmful to honey bee colonies can be different depending on seasonal phases of the honey bee colony size (Table 1). In general, infestation rates reaching 2%–3% should be treated promptly (Honey Bee Health Coalition 2016). Additionally, mite counts on sticky screens from a 10-frame Langstroth hive (single deep and medium super) exceeding 59 mites in 24 hours should also be treated promptly (Delaplane and Hood 1999).
Varroa populations grow quickly throughout the active bee season (typically February through November in Florida). Thus, we recommend that colonies be sampled once per month to determine if infestation rates have reached the treatment threshold. For small beekeeping operations, each colony should be sampled in apiaries with fewer than ten colonies. Larger operations, however, should sample a minimum of eight randomly selected colonies in each apiary (or 3%–5% of all colonies within multiple apiaries) (Honey Bee Health Coalition 2016). It is also a good practice to sample your colonies immediately after treating them for Varroa to confirm treatment efficacy.
How to Monitor Colonies
Varroa population levels in honey bee colonies generally are estimated using two methods:
- Determining the number of mites per 100 bees (infestation rate) within a subsample of adult bees
- Determining the colony Varroa population using natural mite fall
Determining Infestation Rates
Infestation rates are determined by (1) collecting a subset of adult bees into a container, (2) removing the mites from the bees with a dislodging agent, and (3) counting the mites. The industry standard is to collect about 300 bees into the container. Collecting fewer bees reduces the accuracy of the estimate.
The collector divides the number of mites counted by three to estimate the mite infestation rate (number of mites per 100 bees). The beekeeper can use this number and the information in Table 1 to develop and implement an appropriate management strategy for the mite.
Collecting a subset of adult bees into a container:
- Scraping the combs
Materials needed
- A pint (~500 mL) jar with a lid or a Varroa checking container purchased from an equipment supplier.
Preparing the jar
For best results, one should collect at least 300 adult bees from a comb containing emerging brood (Strange and Sheppard 2001; Lee et al. 2010; Dietemann et al. 2013). Be sure not to capture the queen while collecting adult bees from the brood combs. A sample of 300 bees occupies a volume of about ½ cup (100 ml). Mark the container at the ½ cup level so that samples can be taken quickly and efficiently.
Detailed Instructions
- Remove a frame of capped brood from which you see adult bees actively emerging from the cells.
- Hold the frame by the end of the top bar, with the end bar facing up and the frame at a slight angle from vertical.
- Gently scrape the rim of the container down the wax comb, across the adult bees, causing the bees to fall into the container as you progress. If you scrape the container upwards, the bees will not fall back into the container and will likely fly away. Take special care not to collect the queen (Figure 2).
- Tap the container on a hard surface to knock the bees down to the bottom. Continue this process until you have reached the 300-bee mark on the container. Add or remove bees as necessary to reach this mark.

Credit: UF/IFAS Honey Bee Lab
2. Shaking the combs
Materials
- A plastic bin or aluminum baking pan about 9 × 13 inches (23 × 33 cm)
- A pint (~500 mL) jar with a lid, prepared with a 300-bee mark as before
Detailed Instructions
- Remove a frame of capped brood from which you see adult bees actively emerging from the cells.
- Shake bees from the frame into a plastic bin or aluminum baking pan (Figure 3).
- Gently shake the pan to collect all bees into one corner.
- Pour the adult bees into the prepared collection container.
- Tap the container on a hard surface to knock the bees down to the bottom. Continue this process until you have reached the 300-bee mark on the container. Add or remove bees as necessary to reach this mark.

Credit: UF/IFAS Honey Bee Lab
Removing the mites from the bees with a dislodging agent:
We recommend three substances that can be used to dislodge Varroa from adult honey bee samples.
- isopropyl alcohol
- non-sudsy soapy water such as automotive windshield washer fluid
- powdered sugar
None of these substances is distinctly superior to the others at dislodging mites and all are equally reliable (Dietemann et al. 2013). Alcohol and soapy water kills bees while powdered sugar does not. The alcohol and soapy water washes are more labor intensive, but provide a more accurate mite/bee estimate than the powdered sugar shake given that dead bees can be counted. On the other hand, powdered sugar does not kill bees and can be used to estimate Varroa populations quickly in the field. The beekeeper must select a method based on preference and/or convenience. The Honey Bee Health Coalition has produced an informative video demonstrating these sampling techniques.
- Alcohol/Soap Wash
Overview
This method involves adding isopropyl alcohol or soapy water to the container of bees, shaking the container to dislodge the mites, washing the mites from the bees, and counting the mites. This technique gives a more precise measurement of the Varroa infestation rate as the beekeeper can determine the exact number of bees and mites in the sample.
Materials
- A pint (~500 mL) jar, with a lid, containing about 300 bees (collected as mentioned above)
- 1/8 inch (3 mm) screened mesh
- 70% isopropyl alcohol or soapy water
- A white plate or container
Detailed Instructions
- Add the alcohol or soapy water to the jar with bees until ½–¾ of the jar is full (Figure 4).
- Put the lid on the container.
- Shake the container vigorously for 30 seconds (Figure 4).
- Dump the jar's contents onto the 1/8 inch screened mesh suspended over the white plate or container.
- Wash the bees with additional alcohol.
- Count the mites that have fallen from the bees, through the screen, and into the white container.
- Count the number of bees in the sample.
The infestation rate can be determined by dividing the number of mites captured by the number of bees in the sample and multiplying by 100. For example, if you capture 15 mites and that the jar contained 325 bees, the infestation rate would equal the number of mites captured (15) divided by the number of bees in the sample (325) multiplied by 100. The result in this example is 4.6 mites/100 bees, or a 4.6% infestation rate.

Credit: UF/IFAS Honey Bee Lab
2. Powdered Sugar Shake
Overview
This method involves adding powdered sugar to the container of bees, allowing the bees to groom themselves, shaking the mites from the container through a screened lid, and counting the mites. Powdered sugar causes honey bees to groom. This, in turn, causes mites to lose their grip on the honey bees, allowing the former to be shaken from the jar onto a white surface where they can be counted. This nondestructive method does not kill the sampled honey bees, and the bees can be returned to the original colony where the powdered sugar will be removed by other worker bees (Macedo 2002). The disadvantage of this method is that the number of sampled bees is only an estimate. The bees are never counted. Therefore, the mite infestation level is not as accurate when determined this way as it is when determined using an alcohol wash.
Materials
- A pint (~500 mL) jar, with a lid, containing about 300 bees (collected as mentioned above). The lid of the jar must be modified to contain 1/8 inch (3 mm) screened mesh.
- A white plate or container
- Powdered sugar
Detailed Instructions
- Add 2 tablespoons of powdered sugar to the jar with bees. The sugar can be added through the screen mesh (Figure 5).
- Gently shake/roll the jar horizontally so that the powdered sugar is applied evenly to all the bees in the sample.
- Place the jar on a hard surface, in the shade, for 2 minutes.
- Hold the jar upside-down and shake lightly over the white tray for 1 minute (Figure 6).
- Count the mites and record number of mites collected.
- The mite infestation rate can be determined by dividing the number of mites captured by the number of bees in the sample and multiplying by 100, as before.

Credit: UF/IFAS Honey Bee Lab

Credit: UF/IFAS Honey Bee Lab
3. Natural Mite Fall
Honey bees clean themselves (autogroom) or one another (allogroom) of dust, debris, pollen, and even mites. The grooming behavior involves brushing movements of the legs and biting Varroa with their mandibles (Boecking and Spivak 1999). In a natural setting, Varroa may either be groomed off by the bees or naturally fall from the bees or combs through the action of natural hive activity. Consequently, one can sample Varroa by collecting them from below the colony, usually on a sticky board.
Mite fall is a nondestructive and convenient method used to estimate entire colony mite populations, as all bees can be sampled at one time. However, the accuracy of the mite fall method is somewhat questionable because mite fall is largely determined by the amount of emerging brood within a colony (Dietemann et al. 2013). Thus, unless you know your honey bee colony population, you should be cautious about making treatment decisions based on mite fall. In most cases, beekeepers should make treatment decisions based on the infestation rate (mites/100 adult bees), rather than the entire population.
4. Sticky Boards
Overview
Sticky boards can be used to capture mites falling to the bottom of the hive or nest. A sticky board usually is a sheet of stiff cardboard that is sticky on one side. Some may contain a grid to facilitate counting mites (Figure 7). The boards are placed beneath the colony for several days (usually three), following which the mites captured on the board can be counted. This method is the least intrusive of all Varroa quantification methods and is considered a good indicator of overall colony Varroa populations. However, it is the most time consuming of the Varroa monitoring methods because one must insert the screen, remove the screen, and count the mites. You cannot use sticky screens to estimate Varroa infestation rates (number of mites per 100 bees). Instead, you can use the data to estimate whole colony Varroa populations using the formula x = 3.76-y/-0.01 divided by the number of days in a colony, where y represents the total number of Varroa captured on the sticky board and x represents the actual mite population within the colony. (Dr. Keith Delaplane, personal communication).
This can be misleading, though, because you really need an estimate of colony strength to know if the population you determined using sticky boards is harmful to the bees. For example, your screen counts may suggest that you have 3,000 mites in the colony. This would be extremely detrimental to a colony of 10,000 bees, but less so to one with 50,000 bees. That is why we favor making treatment decisions based on the mite infestation rate. That said, we provide sticky board usage information for those who prefer to monitor Varroa levels this way.
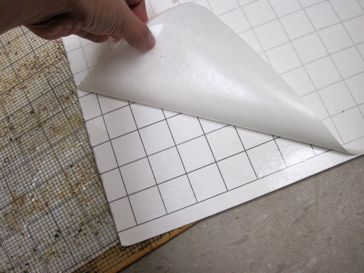
Credit: UF/IFAS Honey Bee Lab
Materials
- Sticky boards (can be purchased from equipment supply companies or made by spreading petroleum jelly on a 13 × 17 inch (33 × 43 cm) white corrugated cardboard)
- A screen to protect the board and keep bees from touching the sticky surface. This can be a screened bottom board or, for hives with a solid bottom board, a "sticky screen," which is a wooden frame containing 1/8 inch (3 mm) mesh screen placed over the sticky board (Figure 8).
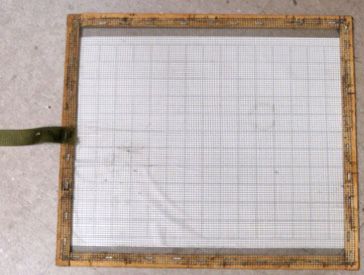
Credit: UF/IFAS Honey Bee Lab
Detailed Instructions
- Place sticky board underneath the colony (or adhere the sheet to the underside of a sticky screen, sliding the entire structure, sticky side up, into the entrance of a hive) (Figure 9).
- Remove the sticky board from the hive after three days (72 hours). Sticky boards left in colonies longer than this can catch a lot of hive debris, making it difficult to count the mites on the board.
- Count the total number of mites found on the sticky board.
- The mite population within a colony can be estimated by substituting the total number of mites captured on a sticky board for y in the equation, solving for x and dividing by the number of days the sticky board was in the hive. For example, if you captured 100 mites on your sticky board after 72 hours, the total colony mite population (x) equals x = 3.76–(100)/-0.01 (3.76–100 = –96.24; –96.24/0.01 mites = 9,624; 9,624/# of days in the hive (3) = 3,208 mites in a colony).

Credit: UF/IFAS Honey Bee Lab
Conclusions
Monitoring Varroa is the foundation of any good strategy for its control. It is necessary to monitor mite population growth to determine when mites have reached a threshold that warrants appropriate treatment. The Varroa quantification methods described in this document will provide beekeepers with practical information about Varroa infestations in their honey bee colonies.
Acknowledgements
Reviews provided by Adam Dale, PhD (agdale@ufl.edu), Entomology and Nematology Department, University of Florida and David Westervelt (David.Westervelt@freshfromflorida.com), chief apiary inspector, Florida Department of Agriculture and Consumer Services.
Selected Resources
Beetsma, J., W. J. Boot, and J. Calis. Invasion behaviour of Varroa jacobsoni Oud.: from bees into brood cells. Apidologie. 1999 30(2–3): 125–140.
Boecking, O., and M. Spivak. 1999. Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 30: 141–58.
Delaplane, K. S., and W. M. Hood. 1999. Economic threshold for Varroa jacobsoni Oud. in the southeastern USA. Apidologie 30: 383–395.
Dietemann, V., F. Nazzi, S. J. Martin, D. L. Anderson, B. Locke, K. S. Delaplane, Q. Wauquiez, C. Tannahill, E. Frey, B. Ziegelmann, P. Rosenkranz, and J. D. Ellis. 2013. Standard methods for varroa research. Journal of Apicultural Research 52: 1–54. doi: 10.3896/IBRA.1.52.1.09
Frey, E., and P. Rosenkranz. 2014. Autumn invasion rates of Varroa destructor (Mesostigmata: Varroidae) into honey bee (Hymenoptera: Apidae) colonies and the resulting increase in mite populations. Journal of Economic Entomology 107: 508–15. doi: https://doi.org/10.1603/EC13381
Honey Bee Health Coalition. 2017. Tools for Varroa management: a guide to effective Varroa sampling and control. Accessed August 8, 2022. https://honeybeehealthcoalition.org/wp-content/uploads/2017/04/HBHC-Guide_Varroa_Mgmt_6thEd_7April2017_c.pdf
Iwasaki, J. M., B. I. P. Barratt, J. M. Lord, A. R. Mercer, and K. J. M. Dickinson. 2015. The New Zealand experience of varroa invasion highlights research opportunities for Australia. Ambio 44(7): 694–704. doi: 10.1007/s13280-015-0679-z
Lee, K. V., R. D. Moon, E. C. Burkness, W. D. Hutchison, and M. Spivak. 2010. Practical sampling plans for Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) colonies and apiaries. Journal of Economic Entomology 103:1039–50. doi: https://doi.org/10.1603/EC10037
Macedo, P. A., J. Wu, and M. D. Ellis. 2002. Using inert dusts to detect and assess Varroa infestations in honey bee colonies. Journal of Apicultural Research 41(2):3–7. doi: 10.1080/00218839.2002.11101062
Rosenkranz, P., P. Aumeier, and B. Ziegelmann. 2010. Biology and control of Varroa destructor. Journal of Invertebrate Pathology 103: S96–S119. doi: https://doi.org/10.1016/j.jip.2009.07.016
Strange, J. P., and W. S. Sheppard. 2001. Optimum timing of miticide applications for control of Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) in Washington State, USA. Journal of Economic Entomology 94(6): 1324–1331. doi: https://doi.org/10.1603/0022-0493-94.6.1324
Thompson, C. E., J. C. Biesmeijer, T. R. Allnutt, S. Pietravalle, and G. E. Budge. 2014. Parasite pressures on feral honey bees (Apis mellifera sp.). PLOS ONE 9:e105164. doi: https://doi.org/10.1371/journal.pone.0105164