The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Sometimes referred to as the mottled water hyacinth weevil, Neochetina eichhorniae Warner (Figure 1) is a weevil that attacks the invasive aquatic plant, water hyacinth, Eichhornia crassipes (Mart.) Solms. Water hyacinth is considered one of the most destructive plants in aquatic ecosystems in the United States and, as a result, is listed on both the federal noxious weed list and Florida's list of prohibited aquatic plants. Neochetina eichhorniae is host specific and causes substantial damage to water hyacinth, making it a valuable biological control agent for this invasive weed in many parts of the world. The insect was first introduced into the United States from Argentina in 1972 when scientists released the insect in Broward County, Florida, to manage water hyacinth (Perkins 1973). Since then, the insect has been introduced in more than three dozen countries worldwide (Winston et al. 2014). Post-introduction studies indicate the insect suppresses the growth of water hyacinth, significantly reducing biomass and flower production (Grodowitz et al. 1991; Center et al. 1999; Tipping et al. 2014; Nesslage et al. 2016; Tipping et al. 2017).

Credit: Georg Goergen, International Institute of Tropical Agriculture (IITA), https://www.flickr.com/photos/iita-media-library/8134250654/
Synonymy
The Integrated Taxonomic Information System (ITIS) lists the following synonym for Neochetina eichhorniae: Neochetina eichborniae Zoological Record, 1979 Missp.
Distribution
Neochetina eichhorniae is native to South America, but over the last five decades has been introduced and established in the United States, Mexico, Australia, and in dozens of other countries in Africa and Asia (Figure 2). In the United States, the establishment of the insect has been confirmed in Florida, Texas, and Louisiana (Winston et al. 2014).
Description
Adults
The adults are mottled dark brown to black and have two parallel tubercles (projections or bumps) on their hardened forewings (elytra), one tubercle on either side of the mid-line (Figure 1). The mottled color pattern is formed by brown, grey, and black scales that densely cover the body of the weevil (O'Brien 1976). The antennae, tarsi (last segment of an insect leg), and apex of tibiae (fourth segment of an insect leg; counted from the body) are usually reddish brown (Figure 1). Males and females can be differentiated based on size of the body and shape of the rostrum (a snout-like projection from the heads of weevils). Males are about 4.1 mm long and have rostrums that are shorter and thicker, weakly curved, and distinctly clubbed on the distal end (apex) (Deloach 1975, O'Brien 1976). In contrast, females are about 4.5 mm long and have rostrums that are longer and moderately slender, are strongly curved with a nearly cylindrical cross section, and uniformly increase in thickness from its base towards the apex (Figure 1, Figure 3, Deloach 1975, O'Brien 1976). Neochetina eichhorniae is superficially similar to Neochetina bruchi Hustache, but the back of Neochetina bruchi is covered by tan scales that form a characteristic V-shaped chevron (Deloach 1975, O' Brien 1976).

Credit: Brandon Woo, https://bugguide.net/node/view/1370808
Eggs
The eggs are long, slender, and flexible. They measure approximately 0.88 mm long by 0.44 mm wide and are typically found beneath the epidermal layer in the leaf blades (lamina) or leaf petioles (the stalk that attaches a leaf blade to the stem) of water hyacinth (Deloach 1975).
Larvae
The larvae have three instars (developmental stages) (Figure 4, Deloach 1975). The width of the head capsule of the larvae increases with each molt, progressing from 0.36 mm for the first instars, 0.56 mm for the second instars, to 0.76 mm for the third instars.
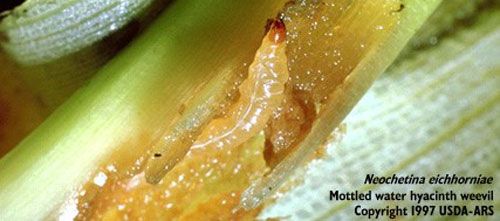
Credit: USDA-ARS
Pupae
The pupae are enclosed in light brown cocoons that appears chitinous. The pupae complete development underwater, sheltered in ball-shaped pupal cases (6.9 mm ± 0.1 mm diameter), among the lateral roots of water hyacinth.
Life Cycle
Before an adult female of Neochetina eichhorniae lays an egg, she uses her mandibles to mine an oviposition hole in the leaf blade or leaf petiole and then uses a side to side movement of her rostrum to deepen the hole (Deloach and Cordo 1976; Stark and Goyer 1983). She then inserts her ovipositor in the oviposition hole and deposits an egg slightly below the epidermal layer (Deloach and Cordo 1976; Stark and Goyer 1983). In a 24-hour period, a female can deposit an average of 2.8 to 7.3 eggs and has been observed to mine up to three extra oviposition holes in which she deposits no eggs, possibly functioning as decoys to confuse predators (Deloach and Cordo 1976; Stark and Goyer 1983). The rate of oviposition depends on factors such as temperature and host plant quality.
Reports from laboratory studies indicate the rate of feeding and oviposition peaks at 30°C (86°F) (Deloach and Cordo 1976) and the average incubation period for eggs is 8.0 (± 0.1) days at 30°C (Deloach and Cordo 1976; Stark and Goyer 1983). Neonates (newly hatched larvae) tunnel throughout the leaf petiole and feed on the internal tissue. As the larvae develop, they progressively tunnel towards the base of the petiole, forming feeding galleries. Second and third instars usually occur singly in feeding galleries, but occasionally two or three feeding galleries merge, causing the larvae to cohabit. Larvae complete development in about 40.5 (± 0.8) days.
In preparation for pupation, the mature larvae (prepupae) exit the feeding galleries and move onto the submerged upper root system of water hyacinth. Each mature larva typically mines the root cortex, creating a lesion (about 1.5 mm long by 0.9 mm wide) on which it forms a ball-shaped pupal case (6.9 mm ± 0.1 mm diameter) by weaving the lateral roots of the plant around itself. The mature larva molts into a pupa within the pupal case. The pupae complete development underwater in about 30 days (Deloach and Cordo 1976).
Teneral (newly eclosed) adults exit the pupal cases and move onto the base of the plant ready to feed, mate, and oviposit, starting a new generation of Neochetina eichhorniae. The newly emerged males and females are usually almost equal in number. Adults are active mostly at night (Stark and Goyer 1983) and live for two to four months (Deloach and Cordo 1976). The insect has been reported to complete three generations per year in Argentina (Deloach 1975) and four generations per year in southern Louisiana (Stark and Goyer 1983). According to studies conducted in Argentina, Neochetina eichhorniae can overwinter as adults, larvae, or pupae (Deloach and Cordo (1976).
Host Damage
The primary host of Neochetina eichhorniae is water hyacinth (Figure 5), an invasive aquatic plant that forms dense monocultures in its invaded range. The plant is native to Brazil but has now invaded freshwater drainage basins in over 50 countries (Figure 6, Winston et al. 2014).

Credit: Katherine Parys, USDA-ARS, Bugwood.org
Credit: Center for Agriculture and Bioscience International (Available online at: https://www.cabi.org/isc/datasheet/20544, Accessed 21 May 2019)
Adults mine the epidermal layer of the leaf and a few cells underneath, creating distinct feeding scars on the leaves (Figure 7, Deloach and Cordo 1976). In a day, an adult can mine approximately 20 feeding scars, which cumulatively equate to about 86 mm2 of leaf surface area. The number of feeding scars per plant is usually directly related to the number of the adults infesting the plant.

Credit: Katherine Parys, USDA-ARS, Bugwood.org
The adults cause damage year-round (Deloach and Cordo 1976). However, larval feeding inflicts the most damage to water hyacinth. The larvae tunnel the leaf petioles and crowns, creating feeding galleries within the plant tissue (Figure 4). Simultaneous attacks by adults and larvae of Neochetina eichhorniae on water hyacinth consequently suppress production of biomass and inflorescences (Bashir et al. 1984, Goyer and Stark 1984, Tipping et al. 2014) and impede growth in established infestations (Forno 1981). Plants under attack by the weevils also are more susceptible to infections by pathogens and loss of buoyancy.
Importance
In the United States, Neochetina eichhorniae together with its congener, Neochetina bruchi, have been credited with reducing the water hyacinth infestation by a third in the Gulf Coast States (Winston et al. 2014). For example, field studies in Texas revealed up to eight Neochetina eichhorniae occurred per plant (Stark and Goyer 1983) and reduced above water biomass of the plant by 57%, below water biomass by 39%, and leaf abundance by 50% (Grodowitz et al. 1991). In Louisiana, an economic analysis covering a 38-year period (1975 to 2013) estimated Neochetina eichhorniae together with other biological control agents reduced the growth rate of water hyacinth by 84% and generated a benefit cost ratio of 34:1 (Nesslage et al. 2016).
In Florida, a field study revealed that, of the three biological control agents released to control water hyacinth as of 2010, Neochetina eichhorniae constituted 99% of all biological control agents found in the field. In the same study, it was reported that water hyacinth infested by the insect had 58.2% less biomass and 97% fewer flowers (Tipping et al. 2014). Reports from countries in Asia, Africa, and in Australia show Neochetina eichhorniae is similarly providing effective control of water hyacinth (Winston et al. 2014). Furthermore, studies have shown that Neochetina eichhorniae can have additive effects when integrated with other control measures such as plant growth retardants (Van and Center 1994) and herbicides (Haag 1986, Tipping et al. 2017).
Monitoring and Management
Samples of water hyacinth plants from an area of interest can be visually examined for the presence of Neochetina eichhorniae adults and larvae, feeding scars on the leaf surfaces (Figure 7), and feeding galleries within the leaves. The number of feeding scars is a reliable indicator of the population intensity of adult Neochetina eichhorniae (Wright and Center 1984). Alternatively, plant samples with suspected infestation can be fragmented by hand and placed in Berlese funnels to extract any larvae or adults of Neochetina eichhorniae feeding on the plant (Tipping et al. 2014). Impact of the insect on the plant can be quantified based on changes in plant parameters such as production of biomass, production of flowers, and density (number of plants per unit area).
Selected References
Bashir MO, El Abjar ZE, Irving NS. 1984. "Observations on the effect of the weevils Neochetina eichhorniae Warner and Neochetina bruchi Hustache on the growth of water hyacinth." Hydrobiologia 110: 95–98.
Center for Agriculture and Bioscience International. 2019. Eichhornia crassipes (water hyacinth). Available online at: https://www.cabi.org/isc/datasheet/20544. (Accessed 21 May 2019)
Center TD, Dray Jr FA, Jubinsky GP, Leslie AJ. 1999. "Water hyacinth weevils (Neochetina eichhorniae and N. bruchi) inhibit water hyacinth (Eichhornia crassipes) colony development." Biological Control 15: 39–50.
Deloach CJ. 1975. "Identification and biological notes on the species of Neochetina that attack Pontederiaceae in Argentina (Coleoptera: Curculionidae: Bagoini)." Coleopterists Bulletin 29: 257–285.
Deloach CJ, Cordo HA. 1976. "Life cycle and biology of Neochetina bruchi, a weevil attacking water hyacinth in Argentina, with notes on N. eichhorniae." Annals of the Entomological Society of America 69: 643–652.
Forno IW. 1981. "Effects of Neochetina eichhorniae on the growth of water hyacinth." Journal of Aquatic Plant Management 19: 27–31.
Goyer RA, Stark JD. 1984. "The impact of Neochetina eichhorniae on water hyacinth in southern Louisiana." Journal of Aquatic Plant Management 22: 57–61.
Grodowitz MJ, Stewart RM, Cofrancesco AF. 1991. "Population dynamics of water hyacinth and the biological control agent Neochetina eichhorniae (Coleoptera: Curculionidae) at a southeast Texas location." Environmental Entomology 20: 652–660.
Haag KH. 1986. "Effective control of water hyacinth using Neochetina and limited herbicide application." Journal of Aquatic Plant Management 24: 70–75.
Nesslage GM, Wainger LA, Harms NE, Cofrancesco AF. 2016. "Quantifying the population response of invasive water hyacinth, Eichhornia crassipes, to biological control and winter weather in Louisiana, USA." Biological Invasions 18: 2107–2115.
O'Brien CW. 1976. "A taxonomic revision of the New World subaquatic genus Neochetina (Coleoptera: Curculionidae: Bagoini)." Annals of the Entomological Society of America 69: 165–174.
Perkins BD. 1973. Release in the United States of Neochetina eichhorniae Warner, an enemy of water hyacinth. Proceedings of the 26th. Annual Meeting Southern Weed Science Society. 368 pp. [Abstract]
Stark JD, Goyer RA. 1983. "Life cycle and behavior of Neochetina eichhorniae Warner (Coleoptera: Curculionidae) in Louisiana: a biological control agent of water hyacinth." Environmental Entomology 12: 147–150.
Tipping PW, Martin MR, Pokorny EN, Nimmo KR, Fitzgerald DL, Dray FA, Center TD. 2014. "Current levels of suppression of water hyacinth in Florida USA by classical biological control agents." Biological Control 71: 65–69.
Tipping PW, Gettys LA, Minteer CR, Foley JR, Sardes SN. 2017. "Herbivory by biological control agents improves herbicidal control of waterhyacinth (Eichhornia crassipes)." Invasive Plant Science and Management 10: 271–276.
Van TK, Center TD. 1994. "Effect of paclobutrazol and water hyacinth weevil (Neochetina eichhorniae) on plant growth and leaf dynamics of water hyacinth (Eichhornia crassipes)." Weed Science 42: 665–672.
Winston RL, Schwarzländer M, Hinz HL, Day MD, Cock MJW, Julien MH. (Eds.) 2014. Biological Control of Weeds: A World Catalogue of Agents and Their Target Weeds, 5th edition, FHTET-2014-04. USDA Forest Service, Forest Health Technology Enterprise Team. Morgantown, WV. 838 pp.
Wright AD, Center TD. 1984. "Predicting population intensity of adult Neochetina eichhorniae (Coleoptera: Curculionidae) from incidence of feeding on leaves of water hyacinth, Eichhornia crassipes." Environmental Entomology 13: 1478–1482.