The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The sheep bot fly, Oestrus ovis L., (Figure 1) is an obligate parasite that is found worldwide (Özdal et al. 2016). It cannot complete its life cycle without utilizing the nasal passages, frontal and maxillary cavities (Allaie et al. 2015), and sinuses of most domesticated sheep, but has been observed in goats. Unlike other flies, females are known to larviposit. This means the eggs are hatched while still inside the female fly, and she will deposit a droplet containing live larvae on the nose of a sheep (Russell et al. 2013).

Credit: Lyle J. Buss, UF/IFAS
The sheep bot fly is generally not a life-threatening parasite, and its presence often goes undetected; however, in rare cases malnutrition or death of the host can occur. In most countries, economic losses due to this pest are not observed, while in others, mainly in tropical and Mediterranean regions, significant losses in productivity occur.
Distribution
Oestrus ovis is found on all six inhabited continents where sheep are tended. Prevalence rates are much higher in Mediterranean and West African countries, including but not limited to Senegal, Italy, Spain, Morocco, and Tunisia (Özdal et al. 2016). The fly can withstand vast differences in temperature, humidity, photoperiod, and elevation with effects principally seen in the number of generations a year, ranging from one to seven generations per year (Yilma and Genet 2000).
Description and Life Cycle
Eggs
The eggs of Oestrus ovis are fertilized, developed, and hatched within a female (Russell et al. 2013).
Larvae
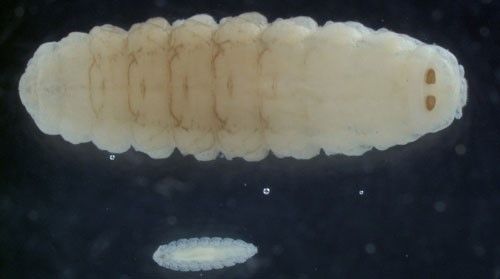
Oestrus ovis is a parasite during the larval portion of its life cycle (Allaie et al. 2015). The larvae of Oestrus ovis develop through three instars, or phases, known as L1, L2, and L3, with L1 being the earliest instar (Figure 2). Most of the fly's life is spent as larval instars, ranging from one to nine months in length depending on the climate and season. Shortest generation times occur in warm, wet climates (Capelle 1966).
Credit: Lyle J. Buss, UF/IFAS
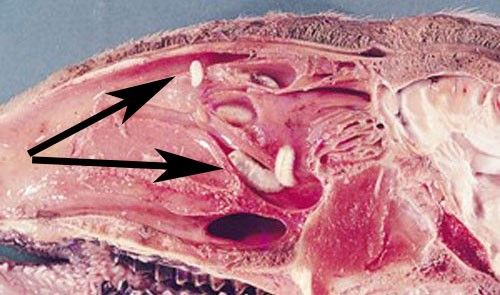
Larvae are deposited in groups of two to 20 on sheep nostrils and develop inside the nose of their host while feeding on the host's mucosal secretions (Figure 3) (Capelle 1966; Russell et al. 2013). The first instars are roughly 0.04 inches (1 mm) long, tapered at the head and posterior ends, composed of 11 body segments, and white in color. They possess two black oral hooks on the head that are used for food acquisition and aiding in locomotion while within the nasal passage. In addition to hooks, larvae also have spines (Figure 4). Four rows of black spines run down their backs. Also, two rounded projections, each with ten curved spines, are located on the posterior segment of larvae (Basmaciyan et al. 2018). The spines' collective function is to help the larvae move quickly up the nasal passages of sheep (Carvalho et al. 2015).
Credit: Jack Lloyd, University of Wyoming

Credit: Lyle J. Buss, UF/IFAS
Once larvae travel through the nasal cavities of the host, molting occurs into the L2 stage. L2 larvae are 0.12 inches (3 mm) to 0.55 inches (14 mm) in length, with physical structures mostly similar to L1. As L2, larvae trigger an immune reaction in their host including an increase of inflammatory cells and parasite-specific immunoglobulins such as IgG and IgA (Silva et al. 2012). To avoid the reaction, larvae move to the nasal sinuses, then molt into L3 stage (Tabouret et al. 2001).
The L3 larvae are cylindrical in shape and transition from yellow-white to brown in color just prior to molting (Figure 5). They are 0.94 inches (24 mm) to 1.18 inches (30 mm) in length with oral hooks that are robust and more sharply curved. When development in this stage is complete, the L3 travel out of the nasal sinuses to the nostrils, where they are expelled by sneezing action of the host (Russell et al. 2013).

Credit: Lyle J. Buss, UF/IFAS
Pupae
Following its expulsion from the host, the L3 transforms into a pupa within 12 to 24 hours. The pupal stage occurs on the soil and inside a puparium, a hardened casing formed from the cuticle of the L3 which encloses a pupa. Within the puparium, the pupa transitions into the adult fly, which emerges after about a month (Basmaciyan et al. 2018). Pupae are brown in color.
Pupae can undergo external hypobiosis, a condition in which they cease development and wait for suitable environmental conditions before emergence from their protective shell. Hypobiosis normally occurs during hot and dry summer months or cold winter months (Tabouret et al. 2001; Özdal et al. 2016).
Adults
Adults range from 0.39 inches (10 mm) to 0.47 inches (12 mm) in length (Abdellatif et al. 2011). Sheep bot fly adults have a dark gray hairy body with dull-yellow head and legs (Figures 1 and 6). The wings are transparent and membranous, normally 0.31 inches (8 mm) in length (Figure 7). Adult flies do not feed, though they may absorb water from their mate while mating. This life stage is relatively short, lasting up to three weeks. During this stage, females produce up to 500 larvae (Capelle 1966).

Credit: Lyle J. Buss, UF/IFAS

Credit: Lyle J. Buss, UF/IFAS
Hosts
The main hosts of Oestrus ovis are sheep, but secondary hosts can include goats or wild ruminants such as deer (Capelle 1966). Sheep bot flies specifically target the noses of their hosts, but occasionally larvae will accidentally enter the eye sockets. This is most likely to happen in animals and humans in close proximity to livestock, particularly domestic sheep. Oestrus ovis infecting humans is rare with only 295 cases reported worldwide between 1918 and 2017 (Basmaciyan et al. 2018).
Sheep bot fly adults prefer dark colored sheep heads over spotted and light colored heads, and there is not a recognized preference between male or female sheep (Özdal et al. 2016). There is no consensus on Oestrus ovis preferring young or old sheep.
Sheep have behaviorally adapted to Oestrus ovis; animals will huddle together with noses pointed to the ground or buried in the wool of others in the flock to protect their noses from female flies. They mainly exhibit this behavior during the hottest part of the afternoon when female flies are most active (Dorchies et al. 1998).
Veterinary Significance
Myiasis is the infestation of animal tissue with fly larvae. Sheep are typically not physically harmed by an infestation of Oestrus ovis. In most cases, animals do not show symptoms of infection (Carvalho et al. 2015); however, clinical signs of infestation in some sheep have included weight loss by up to 9.9 pounds (4.5 kilograms), decreased wool production up to 17.6 ounces (500 grams), and decreased milk production up to 10% (Russell et al. 2013). Damages to animal health can result in economic losses. Additional symptoms of an infestation include sneezing and nasal discharge. In dry, hot climates, dust can accumulate on the nasal discharge of sheep and goats, leading to difficulty breathing (Figure 8) (Dorchies et al. 1998). Irritation of nasal passageways may occur because of the larval spines and oral hooks. Animals can experience hypersensitivity to the presence of the parasite's antigens resulting in an immune response (Tabouret et al. 2001). Also, secondary bacterial infections and major inflammation may arise when larvae die inside the nasal sinuses of sheep (Özdal et al. 2016).

Credit: Jack Lloyd, University of Wyoming
When infesting goats, larvae are less likely to survive than when infesting sheep. In goats, larval survival was 2.2% after two months as compared to 12.7% in sheep. Experts suggest this is because Oestrus ovis may be better adapted to sheep (Dorchies et al. 1998).
Medical Significance
Rarely, Oestrus ovis can be found in and around eyeballs, causing external or internal ophthalmomyiasis, or infection of the eye by larvae. Internal ophthalmomyiasis involves the burrowing of larvae into inner structures of the eyeball, while external ophthalmomyiasis refers to larvae on the conjunctiva, the mucous membrane covering the outer surface of the eye (Sucilathangam et al. 2013). In these cases, larvae do not pass to the L2 stage (Capelle 1966). Larvae cause discomfort and irritation to the eye. In these cases, health professionals will physically extract larvae from the eye through local anesthesia of lidocaine, numbing the area and paralyzing larvae if they are still alive (Russell et al. 2013).
Post-treatment includes antiseptic eye drops, localized pain reliever, and antibiotics. Relief from irritation and healing is rapid. Blindness is extremely rare and usually only associated with internal opthalmomyiasis (Basmaciyan et al. 2018; Yilma and Genet 2000).
Management
Larval infestation of sheep is highest during the spring and summer due to higher temperatures and increased adult fly activity and population size. To combat infestations, producers typically administer chemical treatments to their sheep year-round. Rotation between different treatment products is needed to prevent pesticide resistance from building in Oestrus ovis (Carvalho et al. 2015). Highly successful products work by treating clinical symptoms and preventing the spread of the parasite to other sheep (Tabouret et al. 2001).
Selected References
Abdellatif MZM, Elmazar HMF, Essa AB. 2011. "Oestrus ovis as a cause of red eye in Aljabal Algharbi, Libya." Middle East African Journal of Ophthalmology 18: 305–308.
Allaie IM, Wani ZA, Malik AH, Shahardar RA, Zulhuma M. 2016. "Oestrus ovis larvae in nasal cavity of sheep: A case report." Journal of Parasitic Diseases 40: 1221–1222.
Basmaciyan L, Gabrielle PH, Valot S, Sautour M, Buisson JC, Creuzot-Garcher C, Dalle F. 2018. "Oestrus ovis external opthalmomyiasis: A case report in Burgundy France." BMC Ophthalmology 18: 335.
Capelle KJ. 1966. "The occurrence of Oestrus ovis L. (Diptera: Oestridae) in the bighorn sheep from Wyoming and Montana." The Journal of Parasitology 52: 618–621.
Carvalho RS, Ruivo MA, Colli MHA, Pereira V, Martinez AC, Mazzucatto BC, Cruz BC, Maciel WG, Felippelli G, Teixeira WFP, Soares VE, Costa AJ, Lopes WDZ. 2015. "Occurrences of Oestrus ovis parasitism in necropsied sheep in the Umuarama microregion, Paraná, Brazil." Revista Brasileira de Parasitologia Veterinária 24: 370–374.
Dorchies P, Duranton C, Jacquiet P. 1998. "Pathophysiology of Oestrus ovis infection in sheep and goats: A review." The Veterinary Record 142: 487–489.
Özdal N, Tanritanir P, Ilhan F, Deger S. 2016. "The prevalence of ovine oestrosis (Oestrus ovis Linnaeus, 1761, Diptera: Oestridae) and risk factors in Eastern Turkey." Veterinarski Arhiv 86: 323–333.
Russell RC, Otranto D, Wall RL. 2013. The Encyclopedia of Medical and Veterinary Entomology. ISBN 978-1-78064-037-2. Boston, MA: CAB International pp. 284–287.
Silva B, Bassetto C, Amarante A. 2012. "Immune responses in sheep naturally infected with Oestrus ovis (Diptera: Oestridae) and gastrointestinal nematodes." Veterinary Parasitology 190: 120–126.
Sucilathangam G, Meenakshisundaram A, Hariramasubramanian S, Anandhi D, Palaniappan N, Anna T. 2013. "External ophthalmomyiasis which was caused by sheep botfly (Oestrus ovis) larva: A report of 10 cases." Journal of Clinical and Diagnostic Research 7: 539–542.
Tabouret G, Jacquiet P, Scholl P, Dorchies P. 2001. "Oestrus ovis in sheep: Relative third-instar populations, risks of infection and parasitic control." Veterinary Research 32: 525–531.
Yilma JM, Genet A. 2000. "Epidemiology of the sheep nasal bot, Oestrus ovis (Diptera: Oestridae), in Central Ethiopia." Revue de Médecine Vétérinaire 151: 143–150.