Introduction
Texas root-knot nematode (Meloidogyne haplanaria) was first found attacking peanut in Texas (Eisenback et al. 2003). Texas root-knot nematode is capable of limiting yield of peanut in nematode-infested soils but was less aggressive than the more widely distributed parasites of peanut, Meloidogyne arenaria and Meloidogyne javanica (Bendezu et al. 2004). The peanut cultivar 'NemaTAM', which has been bred for resistance to Meloidogyne arenaria and Meloidogyne javanivca (Simpson et al. 2003), is also resistant to Texas root-knot nematode (Bendezu et al. 2004); however, the resistant cultivars are not widely adopted due to lack of seed supply of the resistant cultivars, lesser yield potential and high susceptibility to Tomato spotted wilt virus (Dong et al. 2008, Grabau et al. 2020). The resistance in tomato to Meloidogyne arenaria, Meloidogyne javanica, and Meloidogyne incognita conferred by the Mi gene is not effective against Texas root-knot nematode (Bendezu et al. 2004). Hence, mitigating the impact of this nematode is essential in peanut and vegetable-producing areas.
Distribution
Texas root-knot nematode is not as widely distributed as some other root-knot nematodes such as Meloidogyne arenaria, Meloidogyne javanica, and Meloidogyne incognita. Since this nematode was first reported in Texas in 2003, it has been reported on tomato in Arkansas (Churamani et al. 2015), tomato and Asian vegetable fields in Florida (Joseph et al. 2016; Bui et. al., 2022), vegetable fields in Georgia (Marquez and Hajihassani, 2023), and pitcher plants in California (Subbotin, 2021). Texas root-knot nematode has not been reported outside of the United States.
Life Cycle and Biology
Accurate identification of root-knot nematodes is essential to efficiently employ management strategies. The major diagnostic criteria to distinguish Meloidogyne haplanaria from other closely related root-knot nematode species are based on morphological differences and host range tests, which are time consuming and labor intensive and require living or well-preserved specimens. Infective juveniles of the Texas root-knot nematode have a body size of 448.2 ± 2.3 µm, stylet length of 12.8 ± 0.3 µm, and tail hyaline length of 16.9 ± 0.2µm. The perineal patterns of Meloidogyne haplanaria showed an extremely rounded and oval shaped pattern (Figure 1).

Credit: Tesfamariam Mengistu, University of Florida
An identification strategy based on mitochondrial DNA analysis has proven useful to identify and address unforeseen plant-parasitic nematode threats to agricultural crops (Pagan et al. 2015). This approach has been used to distinguish morphologically similar but genetically divergent root-knot nematode species as well as new emerging nematode parasites. Texas root-knot nematode and other root-knot nematode species can be identified based on molecular approaches due to the relative simplicity of their application. This identification is done routinely by the University of Florida Nematode Assay Lab based on amplification of two mitochondrial DNA regions. These regions span the intergenic spacer and part of the adjacent large subunit ribosomal RNA gene (lrDNA) of the mitochondrial genome and sequence polymorphisms in mitochondrial lrDNA revealed by the restriction pattern following digestion with the restriction enzymes HinfI and MnlI (Figure 2). A unique haplotype pattern, which has not been observed in any of the root-knot nematode species described thus far, has been observed in Meloidogyne haplanaria.
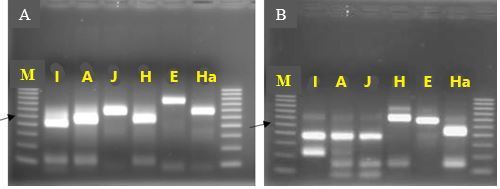
Credit: Soumi Joseph, University of Florida
The Texas root-knot nematode is a sedentary endoparasite that feeds with its body inside the root tissue and whose females are not mobile once they have established a feeding site in the root. While not as well studied as some other species, the life cycle of Texas root-knot nematode is believed to be similar to that of other common root-knot nematodes. Most root-knot nematodes complete their life cycle between 20 to 40 days depending on species, soil temperature, and host suitability. The life cycle begins when females lay eggs into a gelatinous matrix that protects eggs from extreme environmental conditions and parasitism. Development of an embryo starts after egg laying and continues until the juvenile hatches (Figures 3 and 4). Inside the eggs, nematodes develop as first-stage juveniles (J1) and later to second-stage juveniles (J2) and hatch as J2 (Figure 5). Hatching rate of eggs depends on temperature and soil moisture. The J2 are vermiform (worm-shaped) and are the infective stage. After hatching, the J2 must infect a host plant to feed and survive. J2 are attracted to roots of host plants and penetrate root tips with their entire body.
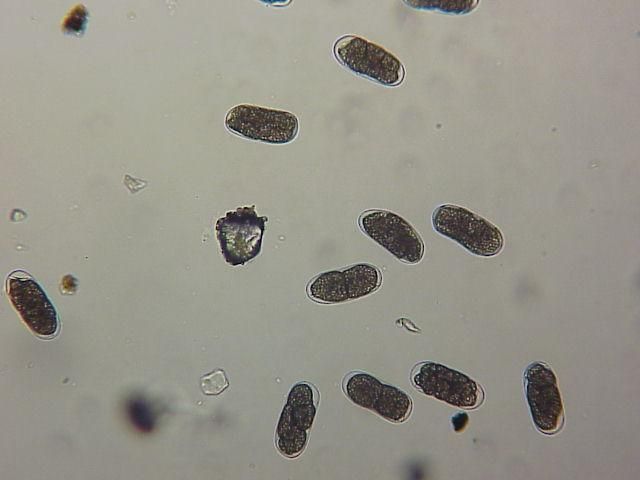
Credit: William T. Crow, UF/IFAS
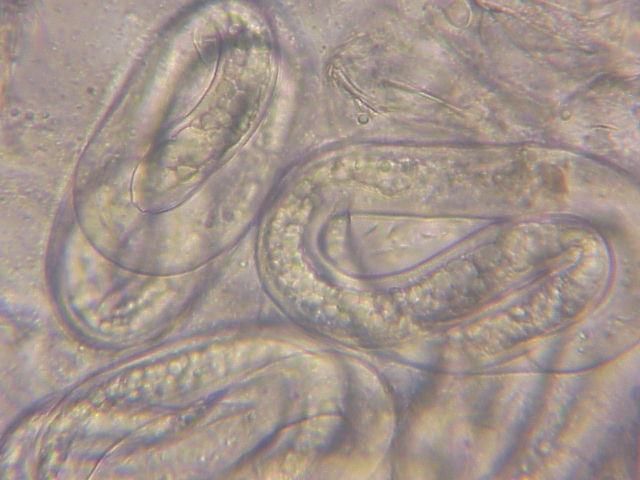
Credit: William T. Crow, UF/IFAS
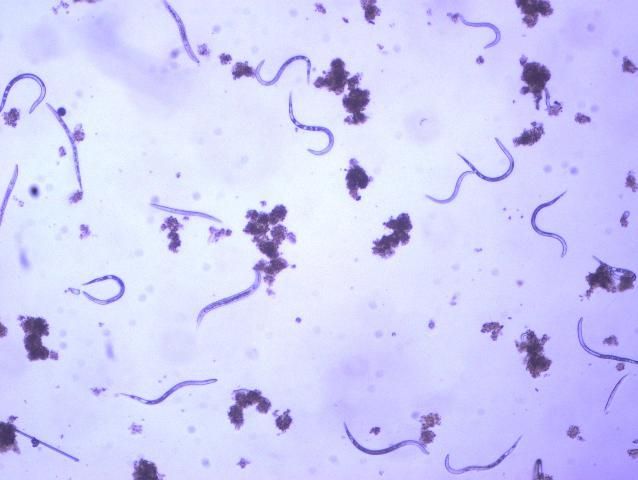
Credit: William T. Crow, UF/IFAS
The J2 migrate within the root to the cortex where they establish their feeding sites called giant-cells (Figure 6). Giant cells are multinucleate cells that act as nutrient sinks, providing a continuous food source for the nematode. After initiating the feeding site, the J2 becomes immobile and its body begins to swell, losing its vermiform shape (Figure 7). The nematode molts into a third (J3) and fourth (J4) stage juvenile, getting larger and more swollen with each molt, and finally into an adult. Adult males are vermiform, exit the root, and no longer feed (Figures 8 and 9). Adult females are swollen, remain sedentary, and continue to feed (Figure 10). Some root-knot nematode species mate, reproduce by amphimixis, and have abundant males; however, most root-knot nematode species, including the Texas root-knot nematode, reproduce without mating by parthenogenesis and males are rare. After laying her eggs in an egg mass, the female dies.
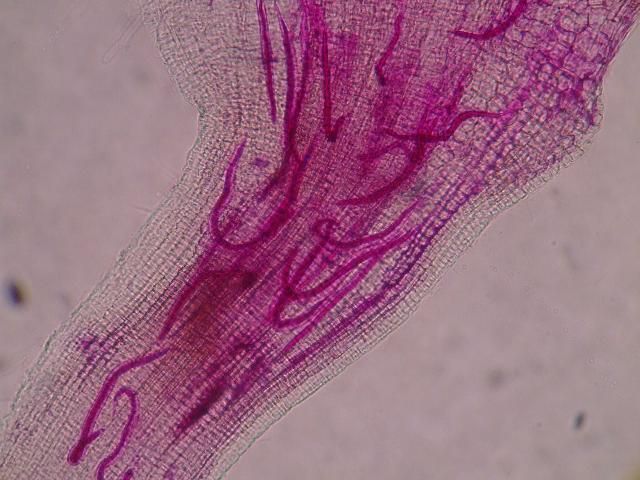
Credit: Nick Sekora, University of Florida
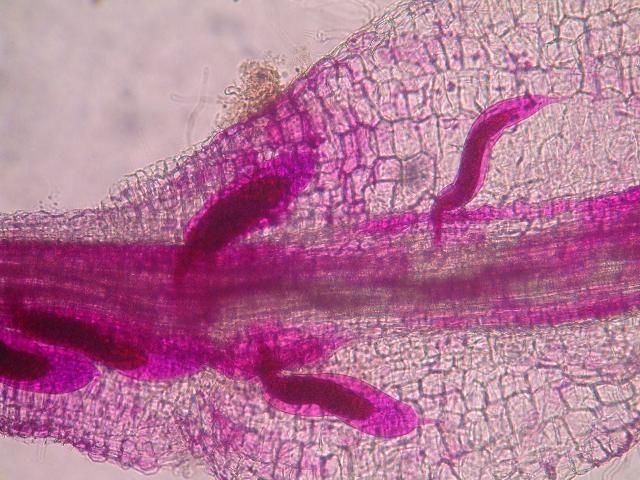
Credit: Nick Sekora, University of Florida
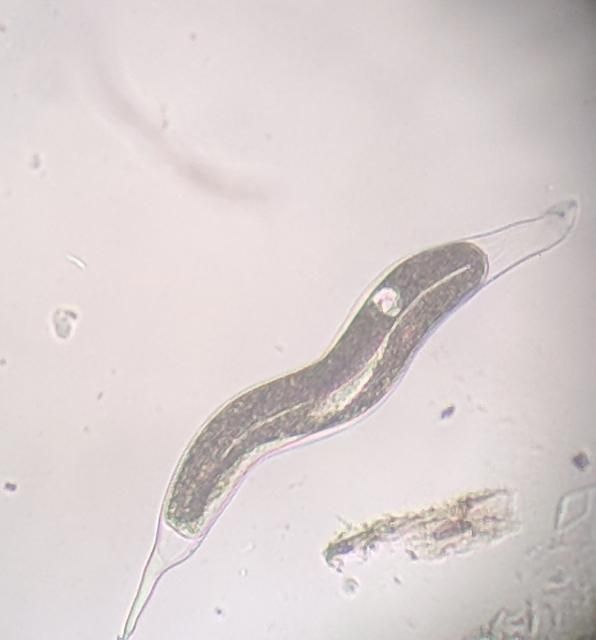
Credit: William T. Crow, UF/IFAS
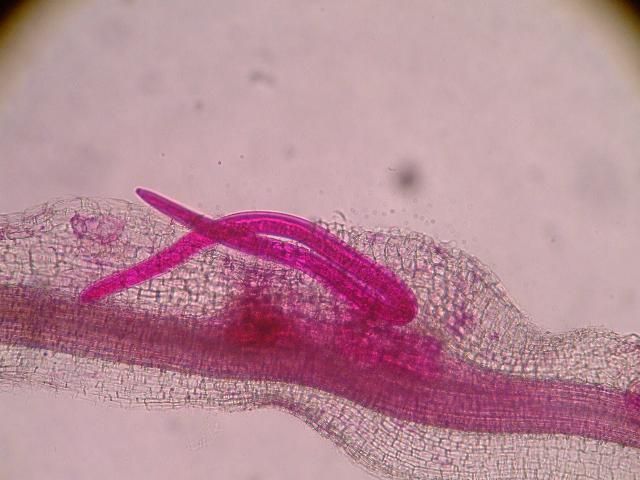
Credit: Nick Sekora, University of Florida
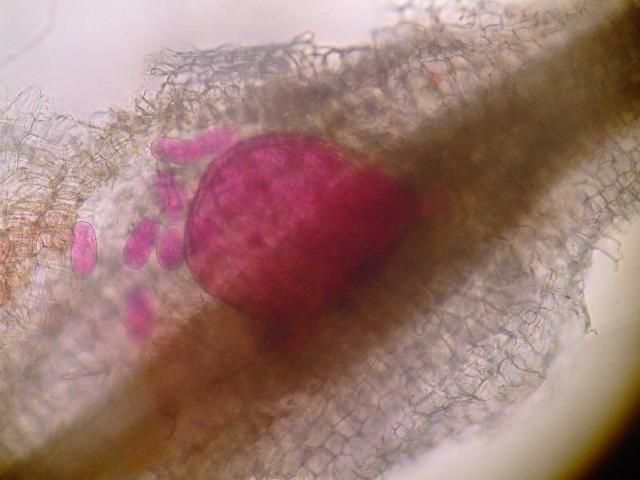
Credit: Nick Sekora, University of Florida
Hosts
Host range studies revealed that Meloidogyne haplanaria can parasitize several legume and crucifer crops including peanut, garden pea, and radish (Eisenback et al. 2003, Bendezu et al. 2004). The Texas root-knot nematode was also reported in Florida causing serious damage to tomato cultivars that are resistant to other root-knot nematode species (Joseph et al. 2016). Its host range is similar to several other root-knot nematode species and is likely to successfully parasitize a large and diverse number of hosts across a large range of plant families.
Economic Importance
Plants affected by the Texas root-knot nematode generally exhibit above-ground symptoms such as decline, stunting, chlorosis, wilting, and yield reductions. In fields, these symptoms are often randomly distributed on small patchy areas (Figure 11); however, symptoms of this nature are not diagnostic, as they may be caused by factors such as other pathogens and nutrient deficiencies. Belowground, plants affected by root-knot nematode exhibit root galling (Figure 12) and egg masses (Figure 13). Galling is easy to see with the naked eye, but egg masses are more difficult to see unless roots are stained or observed under a microscope. It should be noted that galls are a secondary plant response to the nematodes and are not directly involved in feeding or reproduction. The severity of the damage caused by root-knot nematodes will depend on factors such as temperature, initial population density, soil type, moisture, and susceptibility of the host plant. Different root-knot nematode species vary in their ability to gall plants. For example, Meloidogyne hapla usually cause less severe galling on peanut than Meloidogyne haplanaria. On tomato, Meloidogyne haplanaria induced large galls as are typical for Meloidogyne incognita (Figure 12).

Credit: Joe Noling, UF/IFAS

Credit: William T. Crow, UF/IFAS
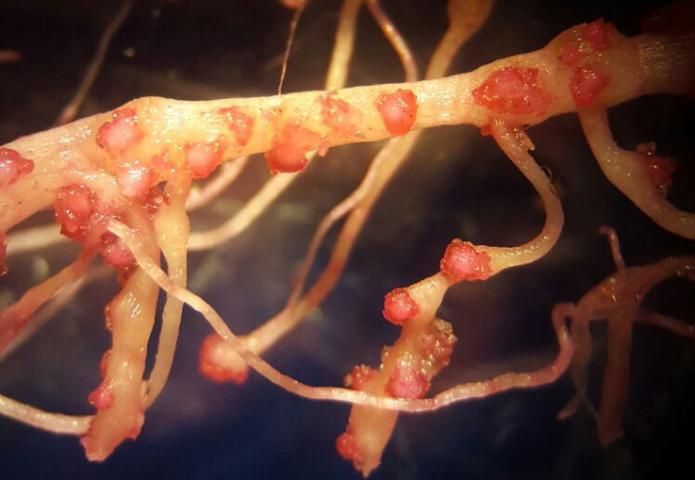
Credit: Tesfamariam Mengistu, University of Florida
Nematode feeding affects yield through different mechanisms such as diversion of plant nutrients to the nematode and reduction in root volume and function. Severe infestations can dramatically reduce yields and eventually kill plants. Damage from Texas root-knot nematode feeding may also increase the incidence of other pathogens like Fusarium oxysporum, a fungus that causes a severe wilt disease of tomato. While either the nematode or the fungus is capable of causing damage individually, much more severe losses are caused when they occur together (Hua et al. 2019).
Management
Owing to their polyphagous nature, management of Texas root-knot nematodes is a difficult task. The Meloidogyne arenaria-resistant peanut cultivar NemaTAM is also resistant to Meloidogyne haplanaria; however, root-knot nematode resistance genes (Mi genes) used in tomato cultivars are ineffective against the Texas root-knot nematode (Bendezu et al. 2004). Pepper was not a host to Texas root-knot nematode (Eisenback et al. 2003). Crop rotation to a non-host such as cotton, pepper, tobacco, or watermelon or a poor host such as corn or wheat, rather than host crops such as tomato, is an effective option for managing Texas root-knot nematode (Eisenback et al. 2003, Bendezu et al. 2004).
For current chemical control and other management options, refer to the appropriate UF/IFAS EDIS Nematode Management Guide chapter for the specific crop and situation. The best management strategy for nematodes is always prevention and to reduce the chance of nematode damage. It is important to take an initial sample to estimate the population densities of plant-parasitic nematodes in the soil. Sampling will provide valuable information regarding the type and number of plant-parasitic nematodes that can harm the crop. For more information about nematode sampling and how to submit a sample, visit the University of Florida Nematode Assay Lab webpage (https://entnemdept.ufl.edu/nematology-assay-lab/).
Selected References
Bendezu F, Morgan E, Starr JL. 2004. "Hosts for Meloidogyne haplanaria." Nematropica 34 (2): 205–209.
Bui HX, Gu M, Riva G, Desaeger JA. 2022. “Meloidogyne spp. infecting Asian vegetables in central Florida, USA.” Nematropica 52:56–63.
Churamani R., McGawley RT, Overstreet, EC. 2015. "Meloidogyne spp. reported from Arkansas: Past and present." Pp. 60, in Proceedings of 54th Annual Meeting of Society of Nematologists. East Lansing, MI.
Dong, WB, Holbrook CC, Timper P, Brenneman TB, Chu Y, Ozias-Akins P. 2008. "Resistance in peanut cultivars and breeding lines to three root-knot nematode species." Plant Disease 92:631–638.
Eisenback JD, Bernard EC., Starr JL, Lee TA, Tomaszewski EK. 2003. "Meloidogyne haplanaria n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitizing peanut in Texas." Journal of Nematology 35(4): 395–403.
Grabau ZJ, Mauldin MD, Habteweld A, Carter ET. 2020. "Nematicide efficacy at managing Meloidogyne arenaria and non-target effects on free-living nematodes in peanut production." Journal of Nematology 52:1–10.
Hua, GKH, Timper P, Ji P. 2019. "Meloidogyne incognita intensifies the severity of Fusarium wilt on watermelon caused by Fusarium oxysporum f. sp. niveum." Canadian Journal of Plant Pathology 41(2): 261–269.
Joseph S, Mekete T, Danquah WB, Noling J. 2016. "First report of Meloidogyne haplanaria infecting Mi-resistant tomato plants in Florida and its molecular diagnosis based on mitochondrial haplotype." Plant Disease 100(7): 1438–1445.
Marquez J, Hajihassani A. 2023. “Identification, diversity, and distribution of Meloidogyne spp. In vegetable fields of South Georgia, U. S. A.” Phytopathology 113(6): 1093–1102.
Pagan C, Coyne D, Carneiro R, Kariuki G, Luambana N, Affokpon A, Williamson VM. 2015. "Mitochondrial haplotype-based identification of ethanol-preserved root-knot nematodes from Africa." Phytopathology 105:350–357.
Simpson CE, Starr JL, Church GT, Burow MD, Paterson AH. 2003. "Registration of 'NemaTAM' peanut." Crop Science 43:1561.