The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Acharia stimulea (Clemens) is a limacodid moth, or slug moth, best known for its larval growth phase. Distinct bright color patterns and the presence of venomous, urticating spines lead to its recognition as the saddleback caterpillar. It is native to a large range in the eastern United States and able to feed on a wide array of host plant species. This species can survive well in northern temperate areas and warmer southern climates. The saddleback caterpillar is encountered most frequently as a medically significant pest, and has minor effects in landscaping and agriculture.

Credit: Lyle J. Buss, University of Florida
Synonymys
Empretia stimulea Clemens
Limacodes ephippiatus Harris
Sibine stimulea (Clemens)
Acharia stimulea (Clemens)
(Dyar and Morton 1896)
Distribution
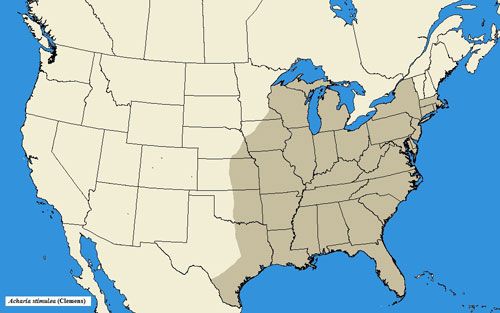
Acharia stimulea has a wide range in the eastern United States, occurring as far southward as Florida, northward to New York and Massachusetts, and westward to Texas, Indiana, and Kansas (Snow 1875; Darlington 1952; Niesenbaum 1992; Landau and Prowell 1999; Heppner 2003; Covell 2005; Wagner 2005).
Description
Adults
Acharia stimulea adults are glossy dark brown in color with black shading. Dense scales are present on the body and wings, giving it a furry appearance. Wing span ranges from 26–43 mm (1.0–1.7 in), with females typically larger in size than males. A single white dot is present near the forewing base. Near the forewing apex, one to three additional white dots are present. The hind wings will be a paler brown than the forewings.

Credit: Lyle J. Buss, University of Florida
Eggs
Clutches are laid on the upperside of host plant leaves in irregular clusters of thirty to fifty eggs. The eggs are sometimes laid to partially overlap each other. Individual eggs are yellow and transparent, with scale-like characteristics and thin edges. Length ranges from 1.5–2.0 mm (0.06–0.08 in) and width averages 1 mm (0.04 in). No additional markings are present.
Larvae
The caterpillars have a truncated, slug-like body form that is not typical of other Lepidoptera families. The prolegs are concealed under the ventral surface of the body. Color patterns are aposematic, or having bright warning colors that denote toxicity or distastefulness. The caterpillars are dark brown on the anterior and posterior ends with a contrasting bright green pattern blanketing the dorsal midsection.

Credit: Lyle J. Buss, University of Florida
This green section is bordered with white markings that outline a saddle-like shape. The skin has a granulated texture, and large fleshy tubercles extend from both anterior and posterior ends. Each tubercle is covered in long setae and a complex of varied urticating spines embedded among the setae.

Credit: Lyle J. Buss, University of Florida
A variable marking occurs at the posterior end where a cream colored set of spots imitates a large facial area.

Credit: Lyle J. Buss, University of Florida
At hatching, larvae can be as small as 1.2 mm (0.05 in) and during last instar the larvae can be as large as 20 mm (0.8 in) with a 7 mm (0.28 in) width. Early instars lack defined aposematic coloration, but still have the stout body design and fleshy tubercles shown by later instars.

Credit: Lyle J. Buss, University of Florida
Middle instars begin to develop the well defined markings of the species and the enlarged spinal processes.

Credit: Lyle J. Buss, University of Florida
Pupae
Larvae spin compact, silk cocoons roughly half the size of the last instar. The cocoon is then hardened to protect the pupa contained within.

Credit: Pennsylvania Department of Conservation and Natural Resources; Bugwood.org
A cocoon from which an adult has emerged will have a circular exit hole as if a hatch has opened. This cocoon design is typical of Limacodidae as a family, and not specific to A. stimulea.
Life Cycle and Biology
The transparent eggs reveal the small larvae of A. stimulea, which are observed curled inside with anterior and posterior ends touching (Dyar and Morton 1896; Epstein 1996; Wagner 2005). After ten days, the minute larva will chew itself free from the egg, creating a small exit hole (Dyar and Morton 1896).
Larvae need approximately four to five months to feed and develop before pupating (Dyar and Morton 1896; Hossler 2010). The first instars lack the aposematic colors or startle display markings that complement their spine defenses. Hatchlings will feed gregariously on a single leaf until advancement to the next growth stage.
The second through penultimate instars acquire brown colorations on the anterior and posterior ends, and develop the bright green saddle markings on the midsection. Although still small at 5–8 mm (0.2–0.3 in) in length, they acquire urticating spines on the fleshy tubercles of the body as early as the second instar. The spinal processes continue to develop and fleshy horns become apparent on the anterior and posterior ends, which function as the primary location of defensive spines. The second to fourth instars feed indiscriminately on the upper or under sides of a host plant leaf, and feed on plant tissue between leaf veins while avoiding the leaf veins themselves; this creates visual dark patches (or 'windows') (Packard 1893; Dyar and Morton 1896).

Credit: Lyle J. Buss, University of Florida
The last instars engorge themselves and reach lengths of approximately 20 mm (0.8 in) with a 7 mm (0.28 in) width. This fully fed stage has noticeably enlarged anterior and posterior ends, with well developed spinal processes and several fleshy horns. The last instars feed on the entire leaf, consuming veins, under side, and upper side regardless of feeding orientation on the leaf (Dyar and Morton 1986).

Credit: Lyle J. Buss, University of Florida
Saddleback caterpillars have modified prolegs where instead of crochets, which hook into materials and surfaces to provide support, the limacodids have suckers (Epstein 1996). Larvae also secrete a semi-fluid silk from their ventral pores as they move. This helps increase body contact with their substrate and provides an adhesive bond, especially to smooth surfaces like those of palm plant leaves (Epstein 1996; Howard et al. 2001). This locomotive complex gives the family its designation as slug caterpillars because of their gliding movement pattern (Epstein 1996).
When on the verge of pupation, limacodid caterpillars expel chalky white frass pellets (Epstein 1996). Fluid is also expelled from the body to reduce the body volume to approximately one half the size of the fully fed larva, at which point the color dulls and pigment is lost (McNaulty 1967). The expelled fluids help compress setae and spines to aid in compaction (Epstein 1996). The silk layer is modified with a circular weakened area at one end (Epstein 1996). This weakened area is not visible before eclosion, and forms a hatch that ejects when pressure is applied by the pupa (Dyar and Morton 1896; Common 1990; Schremmer 1990; Epstein 1996). Despite defensive measures in the cocoon, predation on the pupae accounts for a significant level of mortality in A. stimulea (Murphy and Lill 2010). Caterpillars in temperate areas will overwinter as pupae (USAF 1982; Epstein 1996; Murphy and Lill 2010).
Adults emerge for mating flights in June and July in far northeastern states (Dyar and Morton 1896; USAF 1982), which translates into expected emergences closer to February and March in warmer climate zones as in Texas and Florida. Males fly first in the twilight hours of warm nights, with females following during the night hours; successful couplings will stay in copulatory attachment for up to 24 hours (Dyar and Morton 1896). Adults have a single brood, with oviposition occurring at night. Acharia stimulea is polyphagous, especially on deciduous trees (Scott 1964).
Hosts
What host plant this insect is found on is not a relevant means of identifying A. stimulea because of its polyphagous nature. The following list indicates known hosts:
Aceraceae: Acer spp., maple
Adoxaceae: Viburnum spp.
Anacardiaceae: Schinus terebinthifoilus, Brazilian peppertree
Aquifoliaceae: Ilex cornuta, holly
Araceae: Anthurium andraeanum, flamingo flower; Epipremnum spp., pothos vine; Philodendron selloum
Arecaceae: Areca spp., single stemmed palms; Archontophoenix alexandrae, Alexander palm; Adonidia merrilli, Christmas palm; Caryota mitis, fishtail palm; Chamaedorea spp.; Cocos nucifera, coconut palm; Dictyosperma album, princess palm; Dypsis lutescens, butterfly palm; Nannorrhops ritchiana, mazari palm; Phoenix canariensis, Canary Island date palm; Phoenix roebeleni, pygmy date palm; Syagrus romanzoffianum, queen palm; Washingtonia robusta, Mexican fan palm
Asparagaceae: Beaucarnea recurvata, ponytail palm; Cordyline terminalis, cabbage tree; Dracaena deremensis, green dracaena; D. fragrans cornstalk dracaena; D. marginata, dragon tree
Asteraceae: Aster spp.; Helianthus spp., sunflower and artichoke
Bignoniaceae: Tabebuia aurea Caribbean trumpet tree; T. umbellata Ipê
Cannabaceae: Celtis spp., hackberry
Cannaceae: Canna indica, Indian shot
Cornaceae: Cornus spp., dogwood
Cycadaceae: Cycas revoluta, cycad, sago 'palm'
Ericaceae: Vaccinium spp., blueberry, cranberry, huckleberry, lingonberry
Euphorbiaceae: Acalypha wilkesiana; Codiaeum spp.
Fabaceae: Wisteria spp.
Fagaceae: Castanea spp., chestnut; Quercus spp., oak
Hydrangeaceae: Hydrangea spp.
Iridaceae: Gladiolus spp.
Juglandaceae: Carya illinoinensis, pecan
Lauraceae: Lindera benzoin, spicebush
Leeaceae: Leea coccinea
Lythraceae: Lagerstroemia indica, crape myrtle
Malpighiaceae: Malpighia glabra, Barbados cherry
Malvaceae: Hibiscus rosa-sinensis, Chinese hibiscus; H. syriacus, rose of Sharon; Malvaviscus spp., wax mallow; Tilia spp., basswood
Meliaceae: Swietenia mahogani, West Indies mahogany
Moraceae: Ficus elastica, rubber fig
Myrtaceae: Eucalyptus spp.; Feijoa sellowiana, pineapple guava; Melaleuca quinquenervia, broad leaved paperbark; Psidium cattleianum, strawberry guava; Syzygium spp., brush cherry
Orchiaceae: Cattleya spp., orchid; Chysis bractescens; Cyrtopodium punctatum, cyrt; Dendrobium nobile, noble dendrobium; Epidendrum tampense; Spathoglottis plicata, large purple orchid
Poaceae: Zea mays, sweet corn
Podocarpaceae: Podocarpus spp.
Polygonaceae: Antigonon leptopus, coral vine; Coccoloba uvifera, seagrape
Proteaceae: Macadamia tetraphylla, macadamia nut
Rosaceae: Eriobotrya japonica, loquat; Malus pumila, common apple; M. sylvestris, crabapple; Photinia spp.; Prunus serotina, black cherry; Pyrus spp., pear; Rosa spp., rose
Rubiaceae: Coffea arabica, mountain coffee; Gardenia spp.; Ixora coccinea, jungle geranium
Rutaceae: Citrus aurantium, bitter orange; C. limonia, rangpur; C. paradisi, grapefruit; C. sinensis, sweet orange; Fortunella spp., kumquat
Sapindaceae: Litchi chinensis, lychee or litchi
Sapotaceae: Pouteria campechiana, canistel; Sideroxylon lanuginosum, false buckthorn
Strelitziaceae: Strelitzia nicolai, great white bird of paradise
Ulmaceae: Ulmus spp., elm
Vitaceae: Vitis spp. grapevine
(List compiled using Snow 1875; Darlington 1952; Cassani et al. 1989; Niesenbaum 1992; Howard et al. 2001; Costello et al. 2003; Hepner 2003)
Defenses
The contrasting dark brown ends and bright green saddle are a form of aposematism, which is common among organisms with strong defensive characteristics (Speed and Ruxton 2005). Not necessarily related to aposematism, there are also sets of cream colored spots on the posterior end of the caterpillar that seemingly imitates a face (Dyar and Morton 1986; Packard 1983). These spots are akin to those of other lepidopteran larvae that have more articulate eye spots or snake mimicry in that they confuse aggressors by seeming to have large eyes. These markings discourage predators from attacking by signaling danger (Speed and Ruxton 2005).
The family Limacodidae is also recognized for its members having fleshy horns on the body, each containing numerous hollow spines. In A. stimulea, these hollow spines are present on the edges of the body length, but are concentrated on tuburculated protrusions that adorn both ends of the larvae creating a total of four large horns. Spinose processes vary in shape, length, and articulation relative to where they are on the body, and whether or not multiple types are in proximity to each other (Dyar and Morton 1986; Gilmer 1925; Packard 1983). All of them are capable of breaking away from the body and remaining embedded in contacted surfaces. These spines contain hemolytic and vesicating venom, secreted from nearby glands, that causes direct tissue damage (Scott 1963; Edwards et al. 1986; Murphy et al. 2009).
Saddleback larvae use their great adhesive properties to stay firmly mounted to a surface. When coupled with the venomous spines on the body, this allows the caterpillar to remain attached to any selected surface and offer its spines to aggressors. Sometimes, if disturbed, larvae will arch their back inward in an attempt to create contact with all anterior and posterior horns (Packard 1893; Murphy et al. 2009); however, they are unable to retract the head capsule into the body for protection (Packard 1893).
The aposematic signaling and venomous spines function together to preserve the caterpillar, as the metabolic cost of developing either characteristic is steep. By having both, the organism can enhance the effects of both characteristics and further decrease the probability of aggressive contact (Speed and Ruxton 2005). The large, potent spines of A. stimulea have been shown to effectively deter vertebrate and invertebrate aggressors, with specific interactions showing reduviid bugs, chrysopid lacewings, and vespid wasps avoiding the saddleback caterpillars rather than attacking them (Murphy et al. 2009).
During pupation, the silken cocoon is impregnated with calcium oxalate that is excreted from the maphigian tubules; this hardens the cocoon against disturbance (Ishii et al. 1984). Care should be taken if around a cocoon of Acharia sp., as this genus is documented to weave urticating spines into the silk layer as well (Epstein 1996; Scott 1963).
Medical Significance
Acharia stimulea is best known as a medically significant species. The large spines and potent hemolytic venom rank it as one of the most important North American species of urticating caterpillars, with larvae from the moth family Megalopygidae being the only lepidopterans considered more dangerous (Scott 1963, 1964; Durden and Mullen 2009; Hossler 2010).
The spines of A. stimulea are strong, acutely pointed, and hollow. They embed deeply into tissue and break off, and can interrupt healing as the protoplasm from the venom glands dries into the tissue area (Gilmer 1925). The venom itself can cause a systemic condition called erucism or acute urticaria, for which severe symptoms may include migraines, gastrointestinal symptoms, asthma complications, anaphylactic shock, rupturing of erythrocytes, and hemorrhaging (USAF 1982; Hossler 2009).
Physically manifested symptoms may or may not be present with erucism (Hossler 2009). Contact dermatitis caused by A. stimulea includes immediate intense burning sensations around the contact zone, arector pili muscles tightening causing hair to stand on end, increased perspiration in the affected area, red blanching of the skin, and blistering (Edwards et al. 1986; Hossler 2009, 2010). Symptoms can last for five hours, and leave red blotches in the envenomation site (Hossler 2009).
Prevention of stings is the most effective measure when faced with erucism hazards: protect skin with long sleeves, pants, and gloves when anticipating potential contact with A. stimulea, and avoid areas of heavy infestation if known (USAF 1982; Hesler et al. 1999; Gottschling et al. 2007; Hossler 2009, 2010). Should contact occur, the removal of spines should always take precedence as the longer the spines are embedded in tissue the more venom is administered (Hossler 2009, 2010). Other symptoms should be treated on a case by case basis tailored for your specific reaction by a certified physician (Hossler 2009, 2010).
Personal repellants, such as DEET, are not effective controls for A. stimulea. Manual removal of eggs, larvae or cocoons with proper protective wear and tools is an effective method of control in case of unpredicted population increase (Benaim-Pinto et al. 1991; Gottschling et al. 2007; Iserhard et al. 2007). Spines of urticating caterpillars can become airborne and consequently be inhaled or contact sensitive tissues like the eyes and nose. They may also embed in surfaces such as wooden tables and plastics, which become a contact hazard at a later time if the area is not cleaned. Stray spines can also get caught in fabrics, such as carpet, aprons and clothing and come into skin contact that way (Scott 1963; USAF 1982; Hossler 2009, 2010). If handling urticating caterpillars regularly, such as in rearing facilities, ensure you have access to a frequently sanitized, well ventilated work environment to mitigate hazards of loose spines (Hossler 2009).
Pest Significance
Landscaping
The polyphagous nature of A. stimulea can cause it to be present in a large variety of ornamentals or backyard garden situations (see previous list). Despite this, it is not a significant defoliator of any one plant species (Cassani et al. 1989; Howard et al. 2001; Costello et al. 2003). Acharia stimulea is a venom hazard related to working around plants it may select for oviposition. Wearing appropriate skin covering and manual removal of offenders are options available to those working in the landscape or garden.
Agricultural
As above, the polyphagous nature of A. stimulea has restricted its significance as a defoliator of any one plant. It is interesting to note that A. stimulea is found on more species of palms, including crop palms such as those which produce coconut or oils, than any other limacodid (Howard et al. 2001). Because of the adhesive abilities and strong physical and chemical defenses, A. stimulea is not likely to avoid disturbance or danger, which puts plant handlers at higher risk for contact. The lack of nutrition yielded by palms in particular would also encourage longer feeding intervals by the larvae, leading to higher incidence of contact (Howard et al. 2001). Pest control options such as Bacillus thuringiensis and diflubenzuron have been used to control massive infestations of urticating caterpillars with success (DeLong 1981; USAF 1982; Scholz et al. 1993; Dunlop and Freeman 1997; Hessler et al. 1999; Balit et al. 2001).
Selected References
Balit CR, Ptolemy HC, Geary MJ, Russell RC, Isbister GK. 2001. Outbreak of caterpillar dermatitis caused by airborne hairs of the mistletoe browntail moth (Euproctis edwardsi). Medical Journal of Australia 175: 641–643.
Benaim-Pinto C, Pernia-Rosales B, Rojas-Peralta R. 1991. Dermatitis caused by moths of Hylesia genus (Lepidoptera, Saturniidae) in northeastern states of Venezuela: I. biocenology of Hylesia metabus Cramer. Clinical features of lepidopterism determined by this species. American Journal of Contact Dermatology 2: 213.
Cassani JR, Maloney DR, Habeck DH, Bennett FD. 1989. New insect records on Brazilian peppertree, Schinus terebinthifolius (Anacardiaceae), in south Florida. Florida Entomologist 72: 714–716.
Common IFB. 1990. Moths of Australia. Melbourne University Press, Melbourne, AU. 535 pp.
Costello SL, Pratt PD, Rayamajhi MB, Center TD. 2003. Arthropods associated with above-ground portions of the invasive tree, Melaleuca quinquenervia, in South Florida, USA. Florida Entomologist 86: 300–322.
Covell CV Jr. 2005. A Field Guide to Moths of Eastern North America. Virginia Museum of Natural History, Martinsville, VA. 496 pp.
Darlington EP. 1952. Notes on blueberry Lepidoptera in New Jersey. Transactions of the American Entomological Society 78: 33–57.
DeLong S. 1981. Mulberry tussock moth dermatitis. A study of an epidemic of unknown origin. Journal of Epidemiology and Community Health 35: 1–4.
Dunlop K, Freeman S. 1997. Caterpillar dermatitis. Australasian Journal of Dermatology 38: 193–195.
Dyar HG, Morton EL. 1896. The life-histories of the New York slug caterpillars II. Journal of the New York Entomological Society 4: 1–9.
Edwards EK Jr, Edwards EK, Kowalczyk AP. 1986. Contact urticaria and allergic contact dermatitis to the saddleback caterpillar with histologic correlation. International Journal of Dermatology 25: 467.
Epstein ME. 1996. Revision and phylogeny of the limacodid-group families, with evolutionary studies on slug caterpillars (Lepidoptera: Zygaenoidea). Smithsonian Contributions to Zoology 582: i-iii, 1–102.
Gilmer PM. 1925. A comparative study of the poison apparatus of certain lepidopterous larvae. Annals of the Entomological Society of America 18: 203–239.
Gottschling S, Meyer S, Dill-Mueller D, Wurm D, Gortner L. 2007. Outbreak report of airborne caterpillar dermatitis in a kindergarten. Dermatology 215: 5–9.
Heppner JB. 2003. Arthropods of Florida and Neighboring Land Areas, Volume 17: Lepidoptera of Florida - part 1: Introduction and Catalogue. Florida Department of Agriculture and Consumer Services: Division of Plant Industry, Gainesville, FL. 317 pp.
Hesler LS, Logan TM, Benenson MW, Moser C. 1999. Acute dermatitis from oak processionary caterpillars in a U.S. military community in Germany. Military Medicine 164: 767–770.
Hossler EW. 2009. Caterpillars and moths. Dermatologic Therapy 22: 353–366.
Hossler EW. 2009. Caterpillars and moths Part II: dermatological manifestations of encounters with Lepidoptera. Journal of the American Academy of Dermatology 62: 13–28.
Howard FW, Moore D, Giblin-Davis RM, Abad RG. 2001. Insects on Palms. pp. 33-64. CABI publishing, New York, NY. 400 pp.
Ishii S, Inokuchi T, Kanazawa J, Tomizawa C. 1984. Studies on the cocoon of the Oriental moth, Monema flavescens (Lepidoptera: Limacodidae), 3; structure and composition of the cocoon in relation to hardness. Japanese Journal of Applied Entomology and Zoology 28: 269–273.
Iserhard CA, Kaminski LA, Marchiori MO, Teixeira EC, Romanowski HP. 2007. Occurrence of lepidopterism caused by the moth Hylesia nigricans (Berg) (Lepidoptera: Saturniidae) in Rio Grande do Sul State, Brazil. Neotropical Entomology 36: 612–615.
Landau D, Prowell D. 1999. A partial checklist of moths from mixed mesophytic hardwood forests in Louisiana (Insecta: Lepidoptera). Transactions of the American Entomological Society 125: 139–150.
McNaulty BJ. 1967. Outline of life histories of some West African Lepidoptera, Part II: Limacodidae. Proceedings of the South London Entomological and Natural History Society 1967: 1–12.
Mullen GR, Durden LA. (editors) 2009. Medical and Veterinary Entomology, 2nd edition. Elsevier, Amsterdam. 637 pp.
Murphy SM, Leahy SM, Williams LS, Lill JT. 2009. Stinging spines protect slug caterpillars (Limacodidae) from multiple generalist predators. Behavioral Ecology 21: 153–160.
Murphy SM, Lill JT. 2010. Winter predation of diapausing cocoons of slug caterpillars (Lepidoptera: Limacodidae). Environmental Entomology 39: 1893–1902.
Niesenbaum, RA. 1992. The effects of light environment on growth and herbivory in the dioecious shrub Lindera benzoin (Lauraceae). American Midland Naturalist 128: 271–275.
Packard AS. 1893. The life history of certain moths of the family Cochliopodidae, with notes on their spines and tubercles. Proceedings of the American Philosophical Society 31: 83–108.
Schremmer F. 1990. Das Schlüpfen der Puppe des Asselspinners (Apoda limacodes Hufn.) (Lepidoptera, Limacodidae). Zeitschrift der Arbeitsgemeinschaft Osterreichischer Entomologen 42: 44–52.
Scott HG. 1963. Envenomization. pp. 1-11. United States Department of Health, Education, and Welfare, Atlanta, GA. 18 pp.
Scott HG. 1964. Pest Control's pictorial key of the month: stinging caterpillars. pp. 24-25. United States Department of Health, Education, and Welfare, Atlanta, GA.
Scholz A, Russell R, Geary M. 1993. Investigation of caterpillar dermatitis in school children. New South Wales Public Health Bulletin 4: 65–66.
Snow FH. 1875. Catalogue of the Lepidoptera of eastern Kansas. Transactions of the Kansas Academy of Science 4: 29–63.
Speed MP, Ruxton GD. 2005. Warning displays in spiny animals: one (more) evolutionary route to aposematism. Evolution 59: 2499–2508.
USAF. 1982. Urticating caterpillars.. USAFSAM/EKS, Brooks Air Force Base, TX. pp. 1–3
Wagner DL. 2005. Caterpillars of Eastern North America: a Guide to Identification and Natural History. Princeton University Press, Princeton, NJ. pp. 52.