Introduction
A successful disease control program depends on a crop production system, which closely aligns with the goals of pest management. One must start with the selection of appropriate varieties, an irrigation system that minimizes leaf wetness, a fertilizer program that results in optimal plant growth, plant density and canopy management that afford optimal air circulation and pesticide coverage when needed, a transplant program that minimizes transplant shock, a clean seedling production program, effective pest monitoring by scouting regularly during the season, and, finally, a harvest and shipping procedure that maximizes shelf life and produce quality. Integrated Pest Management (IPM) as applied to vegetable diseases means using all the tactics available to the grower (cultural, biological, host-plant resistance, field scouting, chemical) that provide acceptable yield and quality at the least cost and are compatible with the tenets of environmental stewardship.
The main components of an IPM program are as follows:
-
PREVENTION: Restrict entry of pathogens into fields through planting materials, irrigation water, workers, and tools.
-
MONITORING: Engage in regular field scouting to identify disease symptoms and plant disease vectors. Constantly review pest alerts from diagnostic clinics, state and federal agencies, grower magazines, and bulletins.
-
ACCURATE DISEASE DIAGNOSIS: Consult Extension agents and diagnostic clinics. Identifying the causal organism for a disease is relevant as most biological and chemical control options available are pathogen specific.
-
DEVELOPMENT OF ACCEPTABLE DISEASE THRESHOLDS: Understand the effect of a disease and yield loss. For example, 10% disease incidence because of a specific pathogen may not cause a significant yield loss in a vegetable crop, in which case chemical control may be an unnecessary expense.
-
OPTIMAL SELECTION OF MANAGEMENT TOOLS: Identify an integrated management plan depending upon the disease, crop, and field history. The field history of disease outbreaks is highly relevant in assessing the risk involved in the production. Cultural, host-plant resistance, biological, and chemical control options should be based on the conditions in that specific location.
For disease management, it is important to understand the potential of a pathogen to infect a crop and spread within the crop in a specific region. The three main parameters of disease progress are as follows:
-
INITIAL AMOUNT OF PATHOGEN INOCULUM (INFECTIVE STRUCTURES).
-
RATE OF DISEASE INCREASE.
-
DURATION OF CROP DEVELOPMENT.
These parameters interact to produce a rapid increase in pathogen populations, which manifests as exponential disease development in many production systems.
The rate of disease increase over time is dependent upon the interactions of the pathogen, host plant, and the environment. For disease management purposes, the biggest concern for growers is the interaction of the pathogen and host and the ideal environmental conditions, which plays a critical role in determining the nature of plant disease epidemics. This set of interactions is known as the disease triangle (Figure 1), which determines the fate of a disease on a crop.
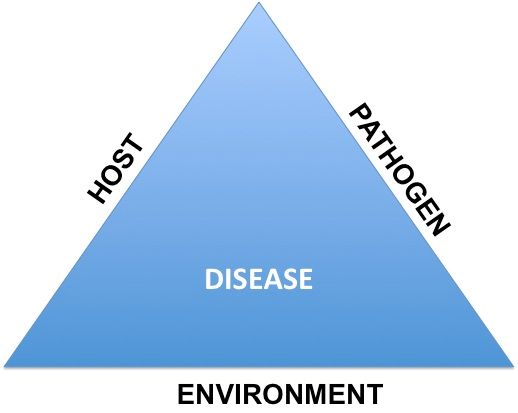
Credit: Mathews Paret
Effective Management Options
-
Understanding the biology of the pathogen, host-pathogen interactions, and the effect of environmental factors on this dynamic process in time and space (disease epidemiology) is critical for planning and implementing effective and efficient management strategies. These strategies can affect particular aspects of the pathogen population's growth. For example, host resistance can affect all disease progress parameters by reducing the amount of inoculum via resistance to particular strains of the pest.
-
Reducing a pathogen's reproductive capacity slows the rate of pathogen buildup.
-
Reducing the total period of exposure in short-season varieties can also be an effective management strategy.
Cultural control practices, however, are aimed at reducing the primary inoculum (sanitation) or reducing the rate of disease increase by modifying the crop environment. A good example of the latter is the use of drip irrigation rather than overhead irrigation to reduce free water on foliage. Biological control usually affects the rate of pathogen buildup. Finally, chemical control can affect the amount of inoculum available at the beginning of the season (i.e., soil fumigation) and/or reduce the rate of disease development by killing a portion of the pathogen involved in later stages of epidemics. IPM combines these practices with pathogen understanding to produce a sustainable and economically beneficial management system. The rest of this document further explains the main concepts of IPM while citing specific examples for its everyday field use.
Accurate Disease Diagnosis
Proper disease identification is critical for making appropriate disease management decisions, and it saves time, money, and the environment. Effective use of fungicides and other pesticides depends on accurate identification of the problem.
The accuracy of any diagnosis depends upon the information supplied, the specimen material selected, and the condition of the specimen when it arrives at a clinic. Digital images of the fresh specimen with symptoms and field-view images of the problem might be useful in some cases. The Distance Diagnostics and Identification System (DDIS) (http://ddis.ifas.ufl.edu/), available through UF/IFAS Extension, may be used for this purpose in many counties.
In order to apply disease management practices, there should be knowledge of which pathogens are present or are likely to appear in a particular field or season. Descriptive and pictorial manuals are helpful for identification of diseases commonly found in Florida. It is important to know the common diseases of a given crop specific to the area. UF/IFAS Extension faculty and professional scouting firms can provide assistance with disease diagnosis. Diagnosis can also be provided by sending samples to the UF/IFAS Plant Disease Clinics, located in Gainesville, Quincy, Wimauma, and Homestead.
Monitoring Pathogens
As mentioned, monitoring is a critical component of an effective IPM program. Monitoring can be direct (looking for the pathogen or disease) or indirect (recording environmental conditions that affect disease development). Financial considerations weigh heavily in the choice of monitoring practices.
Direct disease monitoring can be based on symptoms or signs of the pathogen. Pathogen identification is generally difficult because they are usually microscopic and can be detected typically after the disease process has begun. Most monitoring is actually for disease symptoms, with the control strategy aimed at reducing further spread. Even when visible symptoms are evident, disease levels may be so low as to make detection very difficult. To optimize the chances of detection, one should concentrate on those areas where disease is most likely to occur; for example, low areas or areas of lush growth. If this is not possible, an array of sampling designs may be used, such as a diagonal across the field, a random walk, a stratified design where each subsection of the field is sampled, or a stratified random design where a random sample is taken in each subsection of the field. The appropriate sampling design depends on the level of disease expected, the distribution of the disease, and sampling schemes already in place for other pests.
Disease distribution within a field is dependent, in large part, on the source of inoculum for the pathogen. If the disease is seed-borne, in many cases the first diseased plants are more uniformly distributed in the field. If the disease is soilborne, it may often be found in clusters in the field. If insects transmit the disease, the distribution may be more random, or a field edge effect may be apparent. Thus, it is important to understand the pathogen's biology when developing an appropriate sampling strategy for it.
Indirect disease monitoring most often involves stand-alone computer systems with probes or whole units in the field. Data commonly gathered include temperature, relative humidity, and leaf wetness and can be used to update real-time indices of disease likelihood at a given time. The algorithms for the models are often developed from controlled environmental chamber experiments where the minimum, maximum, and optimal temperatures and relative humidity for fungal growth, germination, and/or disease development are identified. Leaf wetness, either monitored directly or by predicting dew point based on the relative humidity and temperature conditions, is used if the pathogen requires free water for germination. Predicting disease events through environmental monitoring has been very successful in a few cases and is used widely for those crops and diseases where sufficient research exists.
Control Action Guidelines for Diseases
Three major types of models are used for determining when a disease (or vector insect) may exceed its economic threshold, justifying a control action. The simplest is the critical point model, where one parameter is monitored to determine if a disease problem is likely to occur. Perhaps the best known is the model of the corn flea beetle, which transmits the bacterium that causes Stewart's wilt of corn. In this pathosystem, the epidemic is dependent upon the flea beetle vector's ability to survive the winter in the soil. The vector's survival occurs in the northern United States only during mild winters. By adding the average temperatures for December, January, and February, it is possible to predict beetle survival. If the sum of the averages is less than 90, the threat of Stewart's wilt is negligible. Between 90 and 95, the threat is light to moderate. For values between 95 and 100, the threat is moderate to severe. Above 100, the threat is severe.
Although easy to apply, critical point models often are inappropriate because of the complex nature of many epidemics. The multiple point model is used to address more complex pathosystems. These models typically consider a number of parameters and assign severity points to each, with the sum of severity points indicating the potential need for control action. These models are simple to use and do an excellent job of helping to organize what is critical in disease development. An example is the one used by peanut growers in the southeastern United States for tomato spotted wilt (TSW), a disease caused by Tomato spotted wilt virus (TSWV) vectored by thrips species that affects a wide range of crops. Severity points are assigned based upon plant cultivar (the more susceptible, the higher the number), planting date, population density, insecticide use, row pattern (1 or 2 row), and tillage (conventional or strip). The sum of the risk values indicates a low, moderate, or high chance of loss to TSW. Multiple point models can be used in more complex systems but also can be calculated before planting; thus, action can be taken to change the field to a less dangerous situation. For example, if some fields must be planted at a time for optimal disease development, then a more resistant cultivar could be used in those fields.
The most complex type of model is the simulation model. These models often consider environmental conditions in real-time analysis, thus determining when plants are most susceptible to an epidemic. As mentioned above, these models usually require environmental monitoring equipment that record data daily every 15 minutes. The data are input into an algorithm, which determines the risk and length of the conditions for disease development. An inch of rain that occurred over 5 hours has different effects than an inch of rain that occurred over 20 minutes and quickly dried up. These models usually monitor temperature, moisture, dew point, and, sometimes, solar radiation, wind speed, and evaporation potential. The output is similar to simpler models and is usually a range of low to moderate to severe. This information leads to better treatment timing, preventing crop damage and saving sprays. Although simulation models can handle the complexities of a rapidly changing environment, they are usually not as fine-tuned as the multiple point models to account for varieties, planting dates, and other considerations.
Ideally, a single or multiple point model could be applied before the season begins in order to develop the best strategy for that particular growing season. The simulation model can be used to add real-time inputs during the growing season. Such hybrid models are becoming more common as we continue to gain a better understanding of what drives an epidemic.
After the methyl bromide era, a truly integrated approach to soilborne pathogen management is needed. For example, combinations of effective nematicides, herbicides, and fungicides are necessary based on the pest population history of a field or the region's expected soilborne pest problems. Management decisions usually need to be based on pathogen presence/absence before planting. Records of diseases in previous crops, chemical treatments, and weather will no doubt have to be used in disease management decisions. Weather data might be consulted to predict if and when disease outbreaks will occur, providing good experimental data exist to predict likely outbreaks.
Specific Prevention and Management Methods
Site Selection and Preparation: Soilborne diseases remain a major limiting factor for the production of vegetables in Florida. It is important to start with clean soil and proper sites for crops. Plowing and disking reduces pathogen carryover in old crop refuse. The longer the fallow period, the more pathogen populations are reduced. It is also essential to follow the latest recommendations for soil fumigation, cultural practices, and biological control options to eliminate or reduce initial inoculum of soilborne pathogens. It is important to avoid soil compaction because this interferes with root growth, encourages soil moisture retention, and promotes root diseases. Preparation of raised beds generally allows for better drainage. Prior to planting, soil should be tested for nutrient levels and nematode populations (and other pathogens if tests are available). Knowing the history of soilborne disease outbreaks is important for predicting possible future problems. Planting times can be altered to avoid or reduce development of certain diseases.
Host Resistance: It is very important to choose cultivars with multiple pathogen and nematode resistance whenever possible. In Florida, practical control of many diseases of vegetables (Fusarium wilt, Verticillium wilt, and gray leaf spot for tomato) is achieved primarily by this method. Recently, varieties resistant to Tomato yellow leaf curl virus (TYLCV) and TSWV have been identified and should be used in locations that have experienced severe problems in the past.
Irrigation Management: High soil moisture enhances the development of soilborne pathogens, including Phytophthora spp. and Pythium spp. Excess water damages roots by depriving them of oxygen and creating conditions that favor infection by certain soilborne pathogens.
Irrigation management based on plant needs helps create an environment unfavorable for pathogen survival and disease development. Avoiding low areas and using tensiometers or other devices for irrigation scheduling can help in disease management. For UF/IFAS irrigation management recommendations, see https://edis.ifas.ufl.edu/TOPIC_Vegetable_Irrigation.
Soil and Fertilizer Management: Plant nutrition and soil pH can also impact some diseases. Fertilizers with a higher proportion of nitrate nitrogen (NO3) than ammoniacal nitrogen (NH4) help to reduce the incidence of Fusarium wilt on tomato. Increasing soil pH by liming is a good management strategy to reduce Fusarium wilt incidence as well as Botrytis gray mold severity. Optimal calcium nutrition and higher soil pH may reduce the incidence of bacterial wilt in the field. Adequate calcium is necessary to minimize blossom end rot and to provide for overall healthy growth. Avoiding excessive nitrogen leads to less dense canopies, thus improving air movement in the canopy. For UF/IFAS recommendations, see Soil and Fertilizer Management for Vegetable Production in Florida (https://edis.ifas.ufl.edu/cv101).
Cultural Practices: Cultural practices serve an important role in plant disease prevention and management. The benefits of cultural control begin with the establishment of a growing environment that favors the crop over the pathogen. Reducing plant stress through environmental modification promotes good plant health and aids in reducing damage from some plant diseases.
Sanitation practices aimed at excluding, reducing, or eliminating pathogen populations are critical for management of infectious plant diseases. It is important to use only pathogen-free transplants.
In order to reduce dispersal of soilborne pathogens between fields, stakes and farm equipment should be decontaminated before moving from one field to the next. Reduction of pathogen survival from one season to another may be achieved by crop rotation and destroying volunteer plants. Removal of cull piles and prompt crop destruction should be done as general practice.
Avoid soil movement from one site to another to reduce the risk of moving pathogens. For example, sclerotia of Sclerotinia sclerotiorum and Sclerotium rolfsii are transported primarily in contaminated soil. Minimizing wounds during harvest and packing reduces postharvest disease problems. Depending on crops and other factors, soil sanitation can be achieved to some degree by solarization.
Crop rotation is a very important practice, especially for soilborne disease control. For many soilborne diseases, at least a 3-year rotation using a non-host crop greatly reduces pathogen populations. This practice is beneficial for Phytophthora blight of pepper and Fusarium wilt of watermelon, but longer rotation periods (up to 5–7 years) may be needed. Land previously cropped to alternate and reservoir hosts should be avoided whenever possible. Vegetable fields should be located as far away as possible from inoculum and insect vector sources.
Weed control is important for the management of viral diseases. Weeds may be alternate hosts for many important vegetable viruses. Eliminating weeds might reduce primary inoculum. Non-host cover crops help to reduce weed populations and primary inoculum of soilborne pathogens.
Excessive handling of plants, such as in thinning, pruning, and tying, may be involved in spreading pathogens, particularly bacteria. It is advisable to handle plants in the field when plants are driest. Because some pathogens can only enter the host through wounds, situations that promote plant injury should be avoided. During the pruning process and harvest, workers should periodically clean their hands and tools with a disinfectant, such as isopropyl alcohol.
If applicable, plants can be staked and tied for improved air movement in the foliar canopy. A more open canopy results in less wetness, discouraging growth of most pathogens.
Soil aeration and drying can be enhanced by incorporating composted organic amendments in the soil. The pathogen inoculum can be reduced by removing plant material (infected and healthy) after harvest. Between-row cover crops reduce plant injury from blowing sand.
Polyethylene mulch can be used as a physical barrier between soil and aboveground plant parts. This is an important practice for fruit rot control in the field for vegetables. Highly UV-reflective (metalized) mulches repel some insects that transmit viruses as vectors. It is beneficial to use metalized mulch during certain times of the year when insect vectors of some viral diseases are prevalent. TSW incidence and associated thrips populations have been demonstrated to be effectively reduced by using metalized mulches on tomatoes. Metalized mulches cannot be used during winter in southern Florida and early spring in northern Florida because soil temperatures do not reach desirable levels.
Biological Control: The use of biocontrol agents in vegetable disease management is increasing, especially among organic growers. These products are considered safer for the environment and the applicator than conventional chemicals. Examples of commercially available biocontrol agents include the fungi Trichoderma harzianum and Gliocladium virens, an actinomycete Streptomyces griseoviridis, and a bacterium Bacillus subtilis. Bacteriophages (phages) have been found to be an effective biocontrol agent for managing bacterial spot on tomato. Phages are viruses that exclusively infect bacteria. One of the limitations of using biocontrol agents is their inability to survive in certain field conditions. However, biocontrol agents have the ability to improve disease management when integrated with other management options described in this document.
Chemical Control: Fungicides and bactericides are an important component of many disease management programs. It is important to remember that chemical use should be integrated with all other appropriate tactics mentioned in this chapter.
Information regarding a fungicide's physical mode of action helps producers improve fungicide application timing. Physical modes of action of fungicides can be classified into four categories: protective, after infection, presymptom, and antisporulant (postsymptom). Protectant fungicides include the bulk of the foliar spray materials available to producers. In order to be effective, protectant fungicides, such as copper compounds and mancozeb, need to be on the leaf (or plant) surface prior to pathogen arrival. Systemic (therapeutic) fungicides, based on their level of systemic nature (true systemic [i.e., Aliette®], translaminar [i.e., Quadris®], meso-systemic [i.e., Flint®]), are active inside of the leaf (can penetrate at different rates through the cuticle). Systemic fungicides may stop an infection after it starts and prevent further disease development. Fungicides must be used based on recommended fungicide resistance management strategies (https://edis.ifas.ufl.edu/pi131).
A new strategy to chemically manage plant diseases without direct interference with the pathogen is by triggering the plant's defense reaction. Acibenzolar-S-methyl (Actigard®), a chemical in this category, was registered for the control of bacterial spot and speck on tomatoes and is now used commercially.
Chemicals must be used at recommended rates and application frequencies. Besides selection of the most efficacious material, equipment must be properly calibrated and attention must be paid to the appropriate application technique. As always, the key to effective disease management is correct diagnosis of the problem.
Follow the latest fungicide recommendations provided by UF/IFAS Extension publications. Always read the pesticide labels and follow the instructions carefully.
Fumigants can be used to manage soilborne pathogens. Before applying, it is important to review the site's disease history when choosing fumigant materials.
Effective management of whiteflies, thrips, and aphids should be practiced to reduce the incidence and secondary infections of viral diseases vectored by these insects. Apply UF/IFAS recommendations for insect management.