Introduction
Creeping indigo (Indigofera spicata) has reportedly been in Florida for as long as 90 years, but a recent rise in suspected horse poisonings has brought new attention to this toxic plant. Although creeping indigo has been found throughout the state, it is rare or nonexistent on many sites. However, certain heavy traffic areas, such as grass parking lots and/or heavily grazed pastures, may have an abundance of the weed. The low-growing nature of creeping indigo makes it inconspicuous to the casual observer. Fortunately, pasture managers can easily identify the weed during routine scouting once properly trained.
Over the last 10 years, we have seen small outbreaks of creeping indigo poisoning in horses and donkeys in central and north-central parts of the state, especially in areas north of Tampa and around Brooksville. This extension in geographic range of the syndrome appears to correspond to increasing abundance of creeping indigo in the central and north central parts of the state. The origins of this recent invasive wave are unclear. In any event, creeping indigo has become a very common plant around Alachua and Seminole counties and can be found growing along the edges of many pathways, including many at the University of Florida. Even more threateningly, it has become the dominant herb in some pastures in the Ocala and Tampa areas.
Plant Description
Creeping indigo is a prostrate plant (Figure 1) with a very shallow or submerged crown. Leaves are alternate compound and contain 5 to 7 alternate leaflets. Stems grow to 6 feet long and root at the nodes. Flowers (Figure 2) arise from the base of the leaves and contain numerous pink blooms. The plant reproduces by seed, which is the main cause of spread. Seed pods (Figure 3) are needlelike, stiff, approximately 1 inch long, and borne in dense, downward-pointing clusters. They contain 4 to 8 seeds. Leaflets, stems, and seed pods contain numerous appressed hairs (Figure 4). The perennial root is a taproot (Figure 5), which is capable of growing at least 2 feet deep.

Credit: B. Sellers, UF/IFAS

Credit: B. Sellers, UF/IFAS

Credit: B. Sellers, UF/IFAS

Credit: B. Sellers, UF/IFAS

Credit: B. Sellers, UF/IFAS
Signs of Creeping Indigo Toxicity
Consumption of 10 pounds of a different but similar species of Indigofera, I. linneae, daily for 3 weeks (total of 210 pounds of Indigofera) is sufficient to cause the very similar Birdsville disease that is seen in Queensland, Australia (Carrol and Swain 1983; Hopper, Hart, and Smith 1971). Both neurologic and non-neurologic signs are seen. We are uncertain how much creeping indigo a horse needs to consume before clinical signs appear.
Non-Neurologic Signs
There may be weight loss, inappetence, high heart and respiratory rates, labored breathing, high temperature (a rare finding), hypersalivation (ptyalism) or foaming from the mouth, dehydration, pale mucous membranes, feed retention in the cheeks (quidding), halitosis, watery discharge from the eyes (epiphora), squinting (blepharospasm), light sensitivity, corneal opacity, corneal ulceration, and neovascularization, severe ulceration of the tongue and gums (Figure 6), and prominent digital pulses without other signs of laminitis.
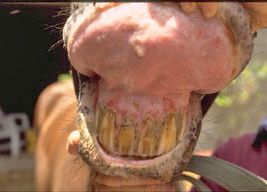
Credit: R. MacKay, UF/IFAS
Neurologic Signs
Often, an early sign is a change in personality—affected horses at first seem quieter and less energetic than usual. Degrees of obtundation (depression) ranging from mild lethargy to recumbency and loss of consciousness may be seen as the condition progresses over days to weeks. Head carriage is low. There may be episodes of standing sleep-like activity (narcolepsy), head-pressing into corners, or compulsive walking around the inside of a stall or paddock. Some affected horses have been seen with their heads tilted to one side and their necks and bodies twisted in the same direction, indicating involvement of the balance centers (vestibular system) of the brain. These signs may be accompanied by rhythmic blinking and jerking eye movements (nystagmus). The blink response to hand gestures toward the eyes (menace response) is frequently absent or reduced, although constriction of the pupils to bright light is usually retained. The muzzle and lips may hang flaccidly. In retrospect, it is often clear that an abnormal gait has been developing over the preceding several days, characterized by incoordination and weakness in all limbs, unpredictable crossing of pairs of limbs, interference between hooves, buckling of joints during weight-bearing, a "crablike" gait, and abnormal posturing at rest. Some affected horses develop a bizarre goose-stepping gait in their front legs. Most horses that continue to consume the plant eventually become cast on their sides and are unable to rise. They either become unconscious or develop convulsions which may become generalized and severe before death or euthanasia after days in recumbency.
Laboratory Findings
Abnormal findings on routine hemograms and plasma chemistry panels are mild, nonspecific, and unhelpful in making the diagnosis. Typically, there is low-normal or low total white blood cell count with lymphopenia and mild electrolyte derangement that may include hyponatremia, hypophosphatemia, hypomagnesemia, and metabolic acidosis. Aspartate aminotransferase and creatine kinase activities often are slightly to moderately above reference range.
Necropsy Findings
Both gross and histologic examinations of horses poisoned by creeping indigo are most notable for the lack of diagnostic findings. Horses may show mild changes in the liver on microscopic examination.
Toxins
Over the last decade, there has been resolution of the previous confusion about the roles of the two putative toxins of creeping indigo, 3-nitropropionate (3-NPA) and indospicine. It is now clear that 3-NPA causes the largely irreversible neurologic signs described above, while indospicine causes the corneal edema, ulcerations, and other non-neurologic signs. Each of these toxins is described briefly:
3-nitropropionate. 3-NPA is a highly toxic compound produced by the plant primarily as defense against destruction by herbivores. This nitrotoxin is by no means unique to Indigofera but is produced as anti-herbivore defense by a vast array of plants and fungi. Poisoning associated with 3-NPA therefore occurs quite commonly in a variety of settings, and the mechanism is well-understood. The toxin is a potent and irreversible inhibitor of mitochondrial succinate dehydrogenase, a key enzyme in transforming glucose and oxygen into usable energy. Nerve cells are extremely vulnerable to energy deprivation, thus accounting for the early and prominent neurologic signs seen with all types of 3-NPA toxicity. 3-NPA accounts for 0.24 to 1.5 percent of the dry matter of creeping indigo. Because it is metabolized quickly, it is unlikely to be found in the serum of affected animals.
Indospicine is a non-protein amino acid. It is toxic to the liver because of antagonism to the essential amino acid arginine, with which it competes. One of its principal toxic actions is inhibition of nitric oxide synthase, an action likely associated with the development of corneal edema and ulceration of mucous membranes. Although horses are relatively resistant to its liver damaging effects, the toxin persists in the tissues of horses dying or killed by the disease, and these tissues are potentially toxic if fed to dogs. Indospicine accounts for 0.1 to 0.5 percent of the dry matter of creeping indigo and it can be detected in the serum of affected animals.
Treatment
Horses that are quickly removed from the offending plants may recover completely, but more often there are persistent gait abnormalities. There is no effective treatment. Early investigations into the prevention and treatment of Birdsville disease in Australia proposed the use of arginine-rich protein sources such as peanut meal (4.3 percent arginine) and gelatin (8.0 percent arginine). The lack of significant liver lesions in horses because of their relative resistance to indospicine, together with the likelihood that the neurological disease results from 3-NPA poisoning, suggests that arginine alone would have little benefit in the treatment of nervous signs, although non-neurologic signs may respond. Thiamine was also suggested as a treatment for nitro toxicity in ruminants, but other studies showed this treatment to be ineffective. The fact that 3-NPA neurodegeneration is used as an induction model for Huntington's disease research is testament to the current futility of all treatments. Management of affected horses should include their removal from the source, confinement to prevent any injuries, and nonspecific supportive therapy. It was previously suggested that livestock poisonings by I. spicata could be prevented by keeping the proportion of the plant below 25 percent of the total forage available, but recent evidence does not support this view. The best way to prevent poisoning is to stop access by horses to paddocks where creeping indigo is present or to remove plants by physical means or herbicide application.
Management
The first step toward managing this weed is to ensure healthy and actively growing pasture grasses (generally bahiagrass). "Pensacola" bahiagrass performs best with a soil pH of 5.5 with regular fertilization. Therefore, it is important to test the soil regularly to ensure that soil nutrient levels are appropriate for optimum bahiagrass growth. When soil sampling, it is also important to submit bahiagrass tissue samples to ensure that adequate phosphorus is present and to generate a phosphorus recommendation when it is deficient in the soil. Please consult EDIS publications on bahiagrass management (Bahiagrass (Paspalum notatum): Overview and Management; https://edis.ifas.ufl.edu/ag342) and tissue testing (Tissue Analysis as a Nutrient Management Tool for Bahiagrass Pastures; https://edis.ifas.ufl.edu/ss475) for additional information on these topics. A second and equally important step is grazing management. As a general rule, a properly managed pasture will support between 500 and 700 pounds of horse per acre. Stocking more horses than this per acre will result in an overgrazed pasture. With time, the pasture grass will start to thin and create an ideal setting for creeping indigo invasion. Therefore, it is important to move the horses to ample land to eliminate overgrazing, or simply remove the animals periodically to allow the pasture to recover. Most pastures perform best if animals are introduced with 6- to 8-inch tall grass, then removed when the grass has been grazed to 3 inches.
Herbicides are an important component of weed management. However, they should be used after soil fertility and grazing management plans are in place. If herbicides are to be used, GrazonNext HL applied at 24 oz/A (1 oz/gallon if spot-spraying) has been shown to be highly effective. This herbicide can be applied to a total of 34 oz per acre per year. Other effective herbicides are metsulfuron and Chaparral (bermudagrass only). Banvel, Weedmaster (or other dicamba + 2,4-D products) and Remedy will also provide some control, but will likely be less effective than the other herbicides mentioned. Refer to product labels for specific instructions concerning each of these herbicides. Additional weed control information for pastures can be found at https://edis.ifas.ufl.edu/wg006.
References
Anderson, R. C., W. Majak, M. A. Rassmussen, T. R. Callaway, R. C. Beier, D. J. Nisbet, and M. J. Allison. 2005. "Toxicity and metabolism of the conjugates of 3-nitropropanol and 3-nitropropionic acid in forages poisonous to livestock." J. Agric. Food Chem. 53: 2344–50.
Borer-Weir, K. E., N. J. Menzies-Gow, S. R. Bailey, P. A. Harris, and J. Elliott. 2013. "Seasonal and annual influence on insulin and cortisol results from overnight dexamethasone suppression tests in normal ponies and ponies predisposed to laminitis." Equine Vet. J. 45: 688–93.
Carroll, A. G., and B. J. Swain. 1983. "Birdsville disease in the central highlands area of Queensland." Aust. Vet. J. 60: 316–17.
Du Puy, D. J., J. N. Labat, and B. D. Schrire. 1993. "The separation of two previously confused species in the Indigofera spicata complex (Leguminosae: Papilionoideae)." Kew Bulletin 48: 727–33.
Emmel, M. W., and G. E. Ritchey. 1941. "The toxicity of Indigofera endecaphylla Jacq. for rabbits." J. Amer. Soc. Agron. 33: 675–77.
Francis, K., C. Smitherman, S. F. Nishino, J. C. Spain, and G. Gadda. 2013. "The biochemistry of the metabolic poison propionate 3-nitronate and its conjugate acid, 3-nitropropionate." IUBMB Life 65: 759–68.
Hegarty, M. P., and A. W. Pound. 1968. "Indospicine: A new hepatotoxic amino-acid from Indigofera spicata." Nature 217: 354–55.
Hopper, P. T., B. Hart, and G. W. Smith. 1971. "Prevention and treatment of Birdsville disease of horse." Aust. Vet. J. 47: 326–29.
Lima, E. F., F. Riet-Correa, D. R. Gardner, S. S. Barros, R. M. Medeiros, M. P. Soares, and G. Riet-Correa. 2012. "Poisoning by Indigofera lespedezioides in horses." Toxicon 60: 324–28.
Morton, J. F. 1989. "Creeping indigo (Indigofera spicata Forsk.) (Fabaceae)—A hazard to herbivores in Florida." Econ. Bot. 43: 314–27.
Ossedryver, S. M., G. I. Baldwin, B. M. Stone, R. A. McKenzie, A. W. van Eps, S. Murray, and M. T. Fletcher. 2013. "Indigofera spicata (creeping indigo) poisoning of three ponies." Aust. Vet. J. 91: 143–49.
Perez, L. B., J. Li, D. D. Lantvit, L. Pan, T. N. Ninh, H. Chai, D. D. Soejarto, S. M. Swanson, D. M. Lucas, and A. D. Kinghorn. 2013. "Bioactive constituents of Indigofera spicata." J. Nat. Prod. 76: 1498–1504.
Schrire, B. 2013. "A review of tribe Indigofereae (Leguminosae-Papilionoideae) in Southern Africa (including South Africa, Lesotho, Swaziland & Namibia; excluding Botswana)." S. Afr. J. Bot. 89: 281–83.
Túnez, I., I. Tasset, V. Pérez-De la Cruz, and A. Santamaría. 2010. "3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington's Disease: Past, present and future." Molecules 15: 878–916.
Wilson, P. G., and R. Rowe. 2008. "A revision of the Indigofereae (Fabaceae) in Australia. 2. Indigofera species with trifoliolate and alternately pinnate leaves." Telopea 12: 293–307.