Introduction
Grunts (family Haemulidae) are some of the most economically and ecologically important fishes found throughout the world. Their common name refers to the characteristic grunting sound they make when they are agitated and during courtship. They rub their pharyngeal teeth together to produce the sound. Grunts are distributed throughout the Atlantic, Indian, and Pacific oceans with 17 genera and approximately 150 species recognized (Nelson 2006). Fifteen species of grunts are common in the western Atlantic and often numerically dominate shallow reefs. Their abundance on coral and rocky reefs, ledges, and hard bottoms has led to a high-value fishery (1.8 million pounds landed in Florida in 2009 [FWRI 2010]). Grunts are primarily harvested by recreational anglers for their value as a food fish.
Many species of grunts are popular in public and private aquariums due to their schooling behavior and bright colors that create interest in aquarium exhibits. The French grunt, Haemulon flavolineatum (Figure 1), has recently been identified as a candidate species for aquaculture due to its popularity in aquarium displays and the development of culture protocols.

Credit: ML Wittenrich
Natural History
The French grunt is an abundant and ecologically important reef fish distributed throughout the Caribbean Sea and portions of the western Atlantic, from Bermuda and South Carolina to the northern Gulf of Mexico (Randall 1967). Adult French grunts typically occur in large resting schools on rocky and coral reefs, often under ledges or large stands of elkhorn coral during daylight hours. Resting schools break up during nocturnal foraging forays on nearby sand flats where they consume mainly crustacean prey such as shrimps, crabs, mantis shrimp, amphipods, and copepods (Hargrove et al. 2012; Burke 1995). Soft-bodied prey such as polychaetes and peanut worms can account for up to 35% of the diet. Juveniles are most common in near-shore seagrass and mangrove areas, feeding in daylight hours on pelagic calanoid copepods, shrimp and crab larvae, amphipods, and Mysis shrimp (Verweij et al. 2006; Lieske and Myers 1994). Larger juveniles migrate to deeper bay and mangrove areas, and subadults (~10 cm) begin to migrate towards deeper hard bottom and reef areas (de la Morinière et al. 2002). Maximum size for French grunts is reported at 30 cm total length, although 17 cm is common.
Despite their high value, little is known about the reproduction and early life history of the group. Settlement of wild French grunt larvae onto reefs has been linked to biweekly moon phase events associated with peak spawning activity (Robertson, Green, and Victor 1988). Spring and summer appear to be peak spawning months in subtropical climates, but at least some spawning is reported to occur year-round, especially in warmer areas.
Culture Techniques
Although limited information is available on the aquaculture methods and systems used to rear French grunts, the University of Florida's Tropical Aquaculture Laboratory (TAL) has developed preliminary aquaculture protocols that will help guide large-scale and continuous production of the species.
Broodstock and Spawning
French grunts reach sexual maturity at 12–15 cm (L. D. Boles, personal observation). In the wild, French grunts spawn at dusk. Male-female pairs rise together in the water column to broadcast their eggs, a behavior that is called a "spawning ascent." At the height of their ascent, the pairs of fish release thousands of separate, spherical, floating eggs measuring slightly less than 1 mm in diameter. To cue cultured French grunts to spawn, light cycle and temperature may be manipulated to simulate spring and summer peak spawning times. Volitional spawning, without the use of hormones, is achieved by maintaining a light cycle of 12–14 hours accompanied by water temperatures of 23°C–28°C and 32–35 g/L salinity. Fecundity is influenced by water temperature with higher temperatures 26°C–28°C maximizing egg output (L. D. Boles, personal observation).
Although several species of grunts are candidates for hormone manipulation of oocyte maturation/ovulation and strip spawning, where male and female gametes are expressed manually and fertilized in seawater, French grunts spawn naturally in relatively small tanks without extensive environmental manipulation. In a study conducted at the Living Seas at Epcot, eight adult French grunts measuring 10 cm fork length were stocked in each of four replicated 660 L (liter) recirculating systems. Within three weeks of stocking, researchers observed spawning in all systems. Photoperiod was maintained at 12 L:12 D, water temperature was 25°C–26°C, and water quality was maintained with wet/dry biomedia and 20-micron cartridge filters.
Once French grunts are acclimated to a captive photoperiod regime, which generally takes two weeks, spawning occurs each night from 30 minutes before to 30 minutes after the lights are turned off. Eggs are spherical and measure 0.89–0.92 mm in diameter (Figure 2). Eggs are buoyant in seawater and collected by means of surface-skimming egg collectors that pass outgoing water through a mesh-screened collection container before traveling to the filtration sump (Cassiano, Ohs, and Hill 2009). Average fecundity is estimated at 6,000–16,000 eggs per female per spawning event.
Broodstock nutrition is known to affect egg and larval quality of some marine fishes. Although untested, it is likely the same for grunts. Developing a broodstock diet that promotes success through hatchery and growout phases is important. Because broodstock diets have not been evaluated for the French grunt, it is recommended that a combination of pelleted feeds (45% protein) be provided in conjunction with fresh or frozen shrimp, squid, clam, and marine fish flesh. Pigfish (Orthopristes chrysoptera) broodstock were fed a similar diet consisting of pellets containing 50% crude protein and 15% crude lipid and frozen squid to initiate spawning (Cassiano, Ohs, and Hill 2009).
Larviculture
French grunt larviculture is relatively straightforward using standard aquaculture techniques, including the use of rotifers and Artemia nauplii as live feeds.
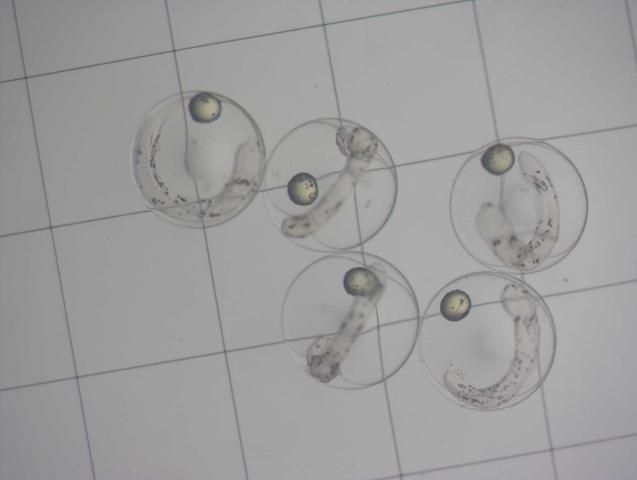
Eggs are removed from the collector the morning after spawning and placed in 1 L glass beakers. Viable, floating embryos can be decanted or pipetted from the surface and separated from dead, sinking embryos and unfertilized eggs after about 10 minutes. Embryos are stocked directly into larval rearing tanks at 15–30 embryos/liter. Larval tanks are maintained between 26°C–28°C and 33–35 g/L salinity. Moderate air flow is provided from an airstone placed in the bottom center of the tank to keep embryos and newly hatched larvae in suspension. Airflow should be sufficient to prevent newly hatched larvae from sinking and settling on the bottom of the tank, which leads to high levels of mortality before the first feeding stage. Hatching occurs within 24 hours after fertilization, and larvae will subsist on yolk sac protein and lipid reserves provided by the broodstock (Figure 3) for the first 2 days at 26°C. During this time, larvae undergo dramatic morphological changes, such as the development and pigmentation of the eyes and development of the mouth and gut as well as the sensory system. They are quite delicate during this stage. Once the yolk is exhausted by the end of the second day, larvae must be provided proper live feeds and the environmental conditions that elicit a feeding reaction.

Larvae have been reared in 13 L, 60 L, and 350 L tanks at the UF/IFAS Tropical Aquaculture Laboratory (UF/IFAS TAL). The influence of background color on feeding success and larval survival has not been evaluated with French grunts. Early larvae did feed well in tanks whose sides and bottoms were black, but tanks of other colors were not evaluated. Neither light intensity nor spectrum were evaluated, but French grunt larvae exhibited a vigorous feeding response under normal fluorescent lamps (6500 K double lamps suspended 10 cm above the surface). At TAL, larval rearing tanks are connected to central filtration systems with mechanical filtration, large fluidized bed sand biological filters, protein skimmers, and UV sterilizers. External standpipes adjust the working volume in each tank and draw outgoing water from the bottom of the tank. Barrel screens placed over the outflow on the bottom of each tank prevent larvae from entering the drain. Tanks are filled with synthetic seawater (Instant Ocean), which is chlorinated (10 mg/L). The water is aged for 48 hours under heavy aeration, and sodium thiosulfate is used to remove residual chlorine one hour before the tanks are stocked. Water is maintained at 26°C–28°C and 33–35 g/L salinity.
First feeding is initiated by the end of the second day when larvae have exhausted their yolk reserves and measure 2.8 mm in length. The airflow is reduced to a gentle flow, and rotifers (Brachionus plicatilis—200 µm lorica length) are stocked at 5–10/mL as an initial diet. A turbidity agent such as algae paste (Nannochloropsis sp.) or live microalgae (Isochrysis galbana, Nannochloropsis sp.) is added at 100,000 to 300,000 cells/mL at this time to provide background contrast that aids in feeding success of larvae.
Aeration is increased slightly at 4 days post hatch (dph), and a slow flow of water from the central filtration system is administered to help reduce nitrogenous waste build-up in the larval rearing tanks. At 10 dph, Artemia nauplii are added at a density of 0.5–1.0/mL. Aeration and water exchange are increased sequentially as the larvae grow in order to maintain high oxygen levels and reduce waste build-up in the tank. Rotifers are removed from the diet by 21 dph. At this time, dry feeds are introduced each morning before Artemia nauplii to facilitate weaning. An equal mixture of Otohime B1 (250–360 µm) and B2 (360–650 µm) dry feed (65% protein, 10% fat) is used during weaning. All fish were weaned to a dry diet by 30 dph. Metamorphosis occurs between 25–30 dph when individuals undergo morphological and behavioral changes that, upon completion, result in juvenile fish (Figure 4).

Studies that have examined the effects of different live prey on the survival of French grunts found 50% survival when fed rotifers and Artemia (Cassiano et al. 2012; Hauville et al. 2017). Survival increased to approximately 70% when French grunts were fed a combination of rotifers and copepod nauplii prior to the Artemia feeding stage. Similar results were observed when examining the larval performance of porkfish (Cassiano et al. 2012).
Grow-Out
Early juveniles can be transferred to grow-out tanks approximately 10 days after weaning to a dry diet, usually by 45 dph. Because early juveniles have high metabolic demands, pelleted feeds of approximately 50% protein should be offered several times throughout the day or offered by means of an automatic feeder. Growth is rapid with juveniles reaching 5 cm by the end of the first month in grow-out. Three-month-old fish measure 6–7 cm.
Market
Market research is needed to fully evaluate the potential market for cultured French grunts. Currently, French grunts are captured by recreational anglers for consumption, used for bait, and used as ornamental fish in the public and private aquarium trade.
Conclusion
The French grunt is well suited to standard aquaculture techniques. The larvae are large at first feeding and readily accept rotifers as an initial diet. Post-metamorphosis growth is rapid, with juveniles reaching two inches (5.08 cm) in just under three months. Furthermore, the relative ease of care in captivity, disposition in community aquarium displays, and striking coloration make the French grunt a good addition to captive display systems, in both public and private aquaria.
Acknowledgements
Special thanks to Rising Tide Conservation and the Association of Zoos and Aquariums for funding this work. Special thanks also to Larry Boles, Andy Stamper, and Stacy Knight of Disney's "The Living Seas" at Epcot, as well as the faculty and staff at the UF/IFAS Tropical Aquaculture Laboratory.
References
Burke, N.C. 1995. "Nocturnal foraging habitats of French and bluestriped grunts, Haemulon flavolineatum and H. sciurus, at Tobacco Caye, Belize." Environmental Biology of Fishes. 42(4): 365–374.
Cassiano, E.J., Ohs, C.L., Hill, J.E. 2009. Candidate species for Florida aquaculture: pigfish, Orthopristis chrysoptera. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/fa160 (accessed June 17, 2013).
Cassiano, E.J., Wittenrich, M.L., Violetta, G.C., Watson, C.A. 2012. "Growth and survival of porkfish (Anisotremus virginicus) larvae: comparing rotifers and copepod nauplii during first feeding." Animal Biology & Animal Husbandry 4(2): 72–78.
de la Morinière, E.C., Pollux, B.J.A., Nagelkerken, I., van der Velde, G. 2002. "Post-settlement life cycle migration patterns and habitat preference of coral reef fish that use seagrass and mangrove habitats as nurseries." Estuarine, Coasta and Shelf Science 55(2): 309–321.
FWRI (Fish and Wildlife Research Institute), Florida Fish and Wildlife Conservation Commission. 2010. Grunts: 86–92. Pdf: http://myfwc.com/media/194701/grunts.pdf
Hargrove, J.S., Parkyn, D.C., Murie, D.J., Demopoulos, A.W., Austin, J.D. 2012. "Augmentation of French grunt diet description using combined visual and DNA-based analyses." Marine and Freshwater Research 63: 740–750.
Hauville, M.R., Cassiano, E.J., Barden, K.P., Wittenrich, M.L., Watson, C.A. 2017. "Larval development, growth and impact of first feed on the aquaculture of French grunt (Haemulon flavolineatum, Desmarest, 1823)." Aquaculture Research DOI: 101111/are.13336
Lieske, E., Myers, R. 1994. Collins pocket guide. Coral reef fishes. Indo-Pacific & Caribbean including the Red Sea. Haper Collins Publishers, 400 p.
Nelson, J. 2006. Fishes of the world (Fourth ed.). John Wiley & Sons, Inc., Hoboken, New Jersey, 601 pp.
Randall, J.E. 1967. "Food habits of reef fishes of the West Indies." Studies in Tropical Oceanography 5: 665–847.
Robertson, D.R., Green, D.G., Victor, B.C. 1988. "Temporal coupling of production and recruitment of larvae of a Caribbean reef fish." Ecology 69(2): 370–381.
Verweij, M.C., Nagelkerken I., Wartenbergh, S.L., Pen, I.R., van der Velde, G. 2006. "Caribbean mangroves and seagrass beds as daytime feeding habitats for juvenile French grunts, Haemulon flavolineatum." Marine Biology 149(6): 1291–1299.