Purpose
The target audience for this publication is current and potential aquaculture producers. The purpose of this publication is to summarize accurate information available about how to culture the almaco jack so that current and potential producers can make informed business decisions.
General Description
Almaco jack, Seriola rivoliana, is a fish within the Order Perciformes, family Carangidae; which includes jacks and pompanos (Figure 1). There is worldwide interest in culturing species in the genus Seriola. Like other commercially farmed Seriola species, almaco jack (also known as longfin yellowtail and Kampachi) are fast growers, have a high market value, and are increasingly well-regarded among chefs for their versatility in both cooked and raw preparations. These characteristics, among others, have made them favorable candidates for both land-based and offshore aquaculture.
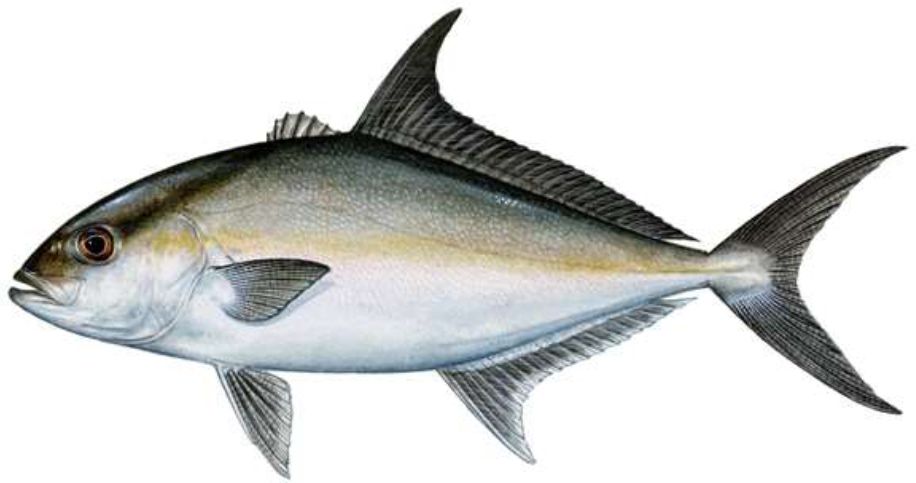
Credit: Copyright Diane Rome Peebles
Almaco jack are characterized by a compressed body, a dark diagonal stripe through the eyes to the nape, the lack of a keel on the ventral side, and a truncated upper jaw with the broad end terminating below, at, or before the anterior margin of the pupil. They can further be differentiated by their darker color and their second dorsal fin being much higher than the dorsal fins of other Seriola spp. In adults, the length of the dorsal-fin lobe is about 1.3 to 1.6 times longer than the pectoral fins and 18% to 22% of the fork length. Generally, they have a brown or silvery blue-green body with faint amber or olive stripes down their sides. The ventral side is much lighter and appears brassy or lavender on adults, whereas the fins and colored bar on the head region are dark and extend from the eye to the rear of the head. Almaco jack is commonly reported to be 90 cm (35 inches) long and to weigh approximately 4.5 kg (10 lbs) (Florida Fish and Wildlife Conservation).
Geographic Distribution and Habitat
Almaco jack (Figure 1) are circumglobally distributed, located in the Gulf of Mexico and intertropical regions throughout the Pacific and Atlantic oceans. They can be found frequenting depths between 3 and 35 m in subtropical regions (43°N–38°S) and on artificial reefs, wrecks, and higher relief structures at depths up to 245 m (800 ft) (Figure 2).
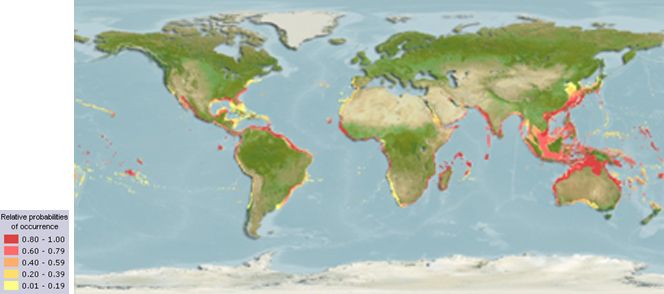
Credit: https://www.aquamaps.org
Culture Methods: Husbandry and System Requirements
Broodstock:
There is little published information on the reproductive biology and spawning of almaco jack in wild populations. Histological studies indicate that ovarian development occurs according to a multiple-batch, group-synchronous pattern similar to greater amberjack Seriola dumerilli (Marino et al. 1995). This means these fish spawn in groups and spawn more than once during the spawning events. Almaco jack spawn from April to November with fish spawning aggregations congregating near the Dry Tortugas, Pulley Ridge, and Flower Garden Banks (Grüss et al. 2018).
Almaco jack and other members of the genus Seriolia have been successfully spawned in captivity using photo-thermal conditioning alone or in combination with the aid of exogenous hormone induction (Rottman et al. 1991). Almaco jack broodstock initially captured from the Gulf of Mexico were conditioned to spawn naturally (26°C; 35 g/L; 12 h light) within 16 weeks of acclimation while being housed in an indoor recirculating aquaculture system. Newly acclimated broodstock were held under photo-thermally controlled conditions in 28 m3 tanks and fed a diet of squid (50%) and Atlantic thread herring (50%) daily at 3% of the total tank biomass (Figure 3). Spawning cohorts with 7 females and 4 to 5 males spawned 3–4 times weekly over three months with an average spawn size of 322,000 eggs with a 58.6% fertilization (Patrick et al. 2019). Similar results were reported using hormonal induction methods (GnRHa, 20 µg/kg) administered to both male and female broodstock, where 10 successful spawns occurred with a mean of 275,000 eggs per spawn with 92% fertilization (Roo et al. 2014). In Hawaii, under natural photoperiod and temperature cycles, F1 broodstock spawned uninterrupted for over two years. Tanks containing 20 adults with 13 spawns/month had a mean fecundity of 154,000 eggs/spawn and mean fertilization of 43% (Laidley et al. 2004). Males were actively producing sperm at 21–22 months of age, whereas females matured more slowly than males, spawning at 24 months of age. Quiñones-Arreola et al. (2015) found that reproductive performance of wild-caught broodstock was better in terms of fertilization rate, total number of spawns, monthly spawn frequency, and total number of eggs produced when compared to first-generation, domesticated broodstock (Table 1).
Table 1. Reproductive performance of wild-caught and first-generation domesticated almaco jack broodstock evaluated for 8 months. (Quiñones-Arreola et al. 2015).
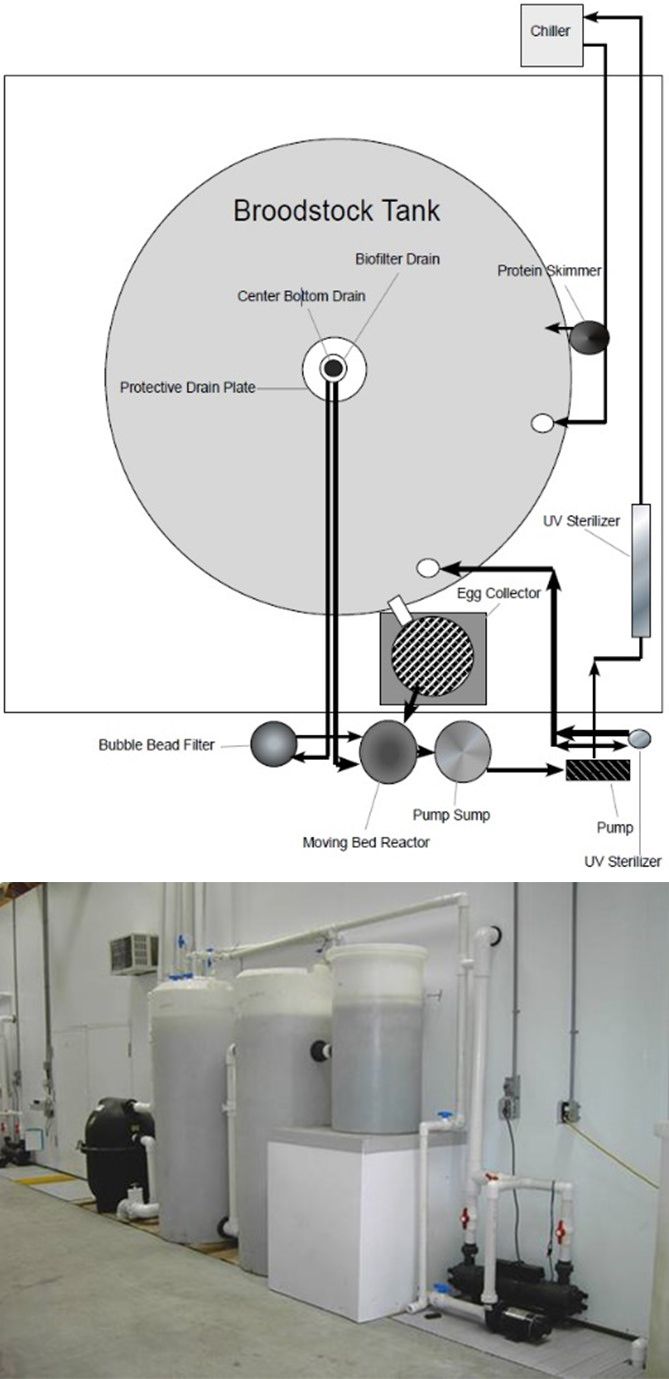
Credit: Victor Blanco, UF/IFAS
Hatchery and Nursery
Hatchery technology for Seriola spp. has progressed over the last two decades with production in Japan, Ecuador, Australia, Chile, Mexico, Europe, and the United States. However, large variations in egg quality and larval survival have been reported for volitional (natural) and hormone-induced spawns, a challenge likely attributed to nutritional deficiencies among broodstock and larval diets ). At 4 days post-hatch, larvae were ready to start first feeding as yolk sac reserves were completely absorbed, eye pigmentation was developed, and the mouth and anus were open (Blacio et al. 2003; Fernández-Palacios et al. 2015; Roo et al. 2014; Roo et al. 2015).
In Ecuador, larval survival in 500 L tanks was reported to be ≤ 2.5% by 21 to 25 days post-hatch (DPH). At 27°C, viable eggs hatched 24 hours post fertilization. Larvae were stocked into 5000 L rectangular tanks. Larvae were fed enriched rotifers and Artemia followed by two dry diets (formulated for trout). Fingerlings weighing approximately 5 g were transferred to outdoor nursery tanks until they reached 10–20 g (Blacio et al. 2003; Laidley et al., 2004).

Credit: Ronald Hans, Mote Marine Laboratory
Larvae cultured in semi-intensive conditions in the Canary Islands had a mean survival of 2.5% at 30 DPH (Roo et al. 2010). At 90 DPH juveniles from semi-intensive systems reached 26.7 ± 4.7 g, while culturing in intensive systems yielded 14.2 ± 5.2 g. A total of 500,000 almaco jack were graded and vaccinated per year with transfer to offshore growout occurring when fish weighed 15-20 g.

Credit: Blue Ocean Mariculture
Tank Growout
Based on gut content analysis, wild adult almaco jack are considered to be almost exclusively piscivorous, however, Ladley et al. (2004) reported the growout of an F1 progeny to maturation in 25 m3 outdoor tanks exclusively feeding a pelleted diet. The fish showed good growth rates and reached weights of over 2 kg in year 1 and almost 5 kg in year 2 (Figure 6). Another study reported initial fish weight (1.76± 0.25 kg), was increased to 6.0 ± 1.1 kg in a thirty-six-month period (Roo et al. 2009). Every year, fish were sampled to measure their individual growth in weight and size (Figure 6).
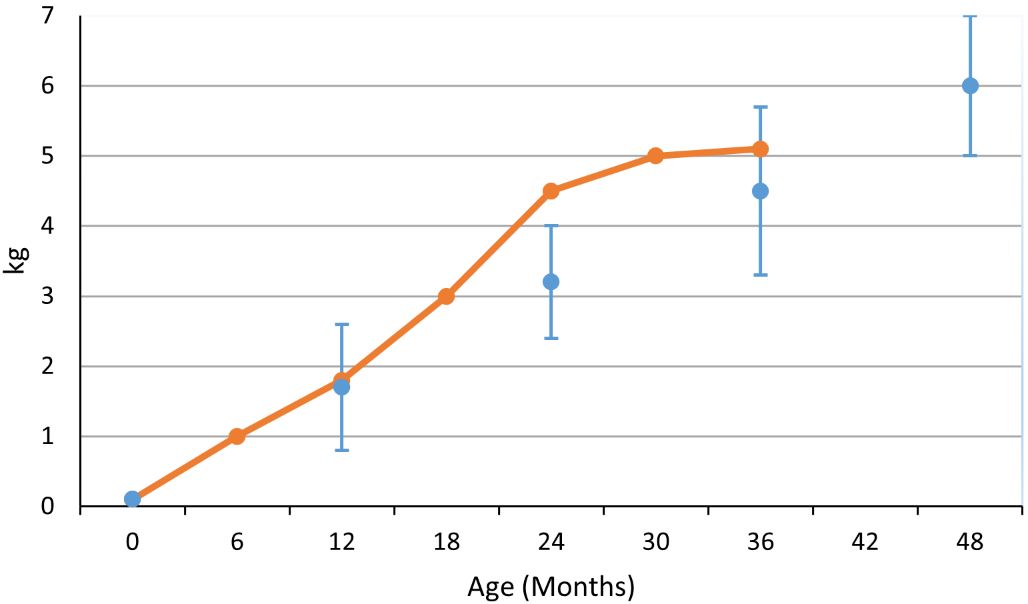
Credit: undefined
Net Pen Growout
In a Hawaiian facility, Benetti et al. (2013) reported that during the husbandry period the operation used 5 SeaStation™ submersible net pens, with up to 8,000 m3 growing volume per net pen. Fish were fed a diet incorporated with a high content of fish meal and taurine. Feed dispersal was achieved via a submerged (water-based) delivery technique. The net pens were harvested twice weekly, and the fresh (non-frozen) product was delivered throughout the United States, with 500+ tons harvested in 2013. To ensure a quality product, H2O2 treatments were required to prevent monogenean skin flukes (Benedenia seriolae).
Current and Potential Markets
Almaco jack is an important member of the Seriola genus, a widely recognized commercial species with an international market. The only domestic commercial aquaculture production to date is in Hawaii, where Blue Ocean Mariculture has been producing hatchery-reared S. rivoliana (branded as Hawaiian Kampachi™) in offshore submersible net pens since 2005. Highly regarded for its flesh quality and high fat content (30%), their product is sashimi-grade and prized for its taste, versatility, and health benefits (Grubman 2014). Almaco jack produced by Blue Ocean Mariculture were cultured in pens for 10–12 months and were harvested at market size of 1.8–2.5 kilograms. The fish then were sent whole to a processor to be distributed through their marketing channels.
Successful worldwide culture of Almaco jack indicates that the species is well suited to captive production. In addition, almaco jack is a hardy species, with a fast growth rate and high market value. These attributes support current research and future interest in the development of almaco jack aquaculture for demanding markets.
References
Abdussamad, E., K. Joshi, and K. Jayabalan. 2008. “Description of Two Lesser Known Jacks of the genus, Seriola (Family: Carangidae) from Indian Waters and Their Comparison with a Closely Related Species, Seriolina nigrofasciata (Ruppell, 1829).” Journal of the Marine Biological Association of India 50 (1): 57–61.
Barreiros, J. P., T. Morato, R. S. Santos, and A. E. S. de Borba. 2003. “Interannual Changes in the Diet of the Almaco Jack Seriola rivoliana (Perciformes: Carangidae) from the Azores.” Cybium 27 (1): 37–40.
Benetti, D., D. Margulies, V. Scholey, J. Wexler, M. Stein, G. Partridge, and J. Stieglitz. 2013. “Overview on Marine Fish Aquaculture in the Americas.” High Value Aquaculture Finfish Symposium, Japan. World Aquaculture Society. https://www.was.org/_documents/kagoshima/8.%20Benetti.pdf
Blacio, E., J. Darquea, and P. Rodríguez. 2003. “Avances en el cultivo de huayaipe, Seriola rivoliana (Valenciennes 1833), en las instalaciones del CENAIM.” El Mundo Acuícola (9):27–28.
Fernández-Palacios, H., D. Schuchardt, J. Roo, C. Hernández-Cruz, and M. Izquierdo. 2015. “Spawn Quality and GnRHa Induction Efficiency in Longfin Yellowtail (Seriola rivoliana) Broodstock Kept in Captivity.” Aquaculture 435:167–172. Elsevier B.V. https://doi.org/10.1016/j.aquaculture.2014.09.021
Gilbert, C. R., and J. D. Williams. 2002. National Audubon Society Field Guide to Fishes. North America. Rev. ed., 2nd ed., fully rev. New York : Alfred A. Knopf, 2002.
Grubman, J. 2014. “Farmed Almaco Jack Seriola rivoliana.” United States Submersible Marine Net Pens. Monterey Bay Aquarium Seafood Watch. 54 pp.
Grüss, A., C. Biggs, W. D. Heyman, and B. Erisman. 2018. “Prioritizing Monitoring and Conservation Efforts for Fish Spawning Aggregations in the U.S. Gulf of Mexico.” Scientific Reports 8 (1): 1–10. https://doi.org/10.1038/s41598-018-26898-0
Laidley, C. W., R. J. Shields, and A. O. Ostrowksi. 2004. “Progress in Amberjack Culture at the Oceanic Institute in Hawaii.” Global Aquaculture Advocate 7:42–43.
Marino, G., A. Mandich, A. Massari, F. Andaloro, S. Porrello, M. G. Finoia, and F. Cevasco. 1995. “Aspects of Reproductive Biology of the Mediterranean Amberjack (Seriola dumerilii Risso) during the Spawning Period.” Journal of Applied Ichthyology. https://doi.org/10.1111/j.1439-0426.1995.tb00002.x
Patrick, G., A. M. Tarnecki, N. Rhody, R. Schloesser, K. Main, R. Yanong, and R. Francis‐Floyd. 2019. “Disinfection of Almaco Jack (Seriola rivoliana Valenciennes) Eggs: Evaluation of Three Chemicals.” Aquaculture Research 50 (12): 3793–3801. https://doi.org/10.1111/are.14342
Quiñones-Arreola, M. F., G. Fabiola Arcos-Ortega, V. Gracia-López, R. Casillas-Hernández, C. Weirich, T. Morris, M. Díaz-Tenorio, and C. Ibarra-Gámez. 2015. "Reproductive Broodstock Performance and Egg Quality of Wild-Caught and First-Generation Domesticated Seriola rivoliana Reared under Same Culture Conditions.” Latin American Journal of Aquatic Research 43:953–962. https://doi.org/10.3856/vol43-issue5-fulltext-15
Roo, J.; Schuchardt, D.; Socorro, J.; Guirao, R.; Hernández C., Fernández, H. 2009. Maduración y obtención de puestas de Seriola rivoliana en Canarias. XII Congreso Nacional de Acuicultura: Libro de Resúmenes. Madrid, España. pp 598–599.
Roo, J.; Grossi, E.; Schuchardt, D.; Socorro, J.; Hernández, C.; Izquierdo, M.; Fernández, H. 2010. “Potential of Almako Jack, Seriola rivoliana, as a Fast-Growing Species for European Aquaculture Diversification.” Universidad de Las Palmas de Gran Canaria. https://www.researchgate.net/profile/Javier_Roo/publication/267024778_POTENTIAL_OF_ALMAKO_JACK_Seriola_rivoliana_AS_A_FAST-GROWING_SPECIES_FOR_EUROPEAN_AQUACULTURE_DIVERSIFICATION/links/5441089a0cf2e6f0c0f4e37a.pdf
Roo, J., H. Fernández-Palacios, C. M. Hernández-Cruz, A. Mesa-Rodriguez, D. Schuchardt, and M. Izquierdo. 2014. “First Results of Spawning and Larval Rearing of Longfin Yellowtail Seriola rivoliana as a Fast-Growing Candidate for European Marine Finfish Aquaculture Diversification.” Aquaculture Research 45 (4): 689–700. https://doi.org/10.1111/are.12007
Roo, J., H. Fernández-Palacios, D. Schuchardt, C. M. Hernández-Cruz, and M. S. Izquierdo. 2015. "Influence of Hormonal Induction and Broodstock Feeding on Longfin Yellowtail Seriola rivoliana Maturation, Spawning Quality and Egg Biochemical Composition.” Aquaculture Nutrition 21 (5): 614–624. https://doi.org/10.1111/anu.12188
Rottman, R.W., J.V. Shireman, and F.A. Chapman. 1991. “Introduction to Hormone-Induced Spawning of Fish.” Southern Regional Aquaculture Center, Publication No. 421. 4 p.
Sicuro, B., and U. Luzzana. 2016. “The State of Seriola spp. Other Than Yellowtail (S. quinqueradiata) Farming in the World.” Reviews in Fisheries Science and Aquaculture 24:314–325. Taylor & Francis. https://doi.org/10.1080/23308249.2016.1187583
Sims, N., L. Vollbrecht, and D. Peters. 2019. “The Velella Epsilon Project: Pioneering Offshore Aquaculture in the Southeastern Gulf of Mexico.” Florida Sea Grant Pioneering Offshore Aquaculture Workshop. https://www.flseagrant.org/aquaculture/openocean/pioneering-offshore-aquaculture-workshop/
Smith-Vaniz, W. F. 2003. “Jacks and Scads (Bumpers, Pompanos, Leatherjacks, Amberjacks, Pilotfishes, Rudderfishes).” The Living Marine Resources of the Western Central Atlantic, Volume 3: Bony fishes part 2 (Opistognathidae to Molidae):1426–1468.