The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Linepithema humile (Mayr) (Figure 1), commonly referred to as the Argentine ant, is native to South America but is globally distributed and is an invasive species in many parts of the world. While it does not sting or bite humans, the Argentine ant is a nuisance pest in urban areas and will enter homes in search of food and water. It is also considered a pest of ornamental and crop plants primarily due to its feeding habits of tending and protecting honeydew producing insects, such as aphids and scale insects. This aggressive species, capable of producing numerous workers, also has a negative effect on native ant species. Linepithema humile colonies have multiple queens, allowing colonies to grow and spread rapidly. Linepithema humile has been designated as a tramp ant species (McGlynn 1999). Like other tramp species, Linepithema humile can establish in new locations quickly due to the multi-queen nature of the colonies, their ability to forage and find resources, and the extensive network of interrelated colonies they are able to form.

Credit: Philip Herbst. From www.antweb.org
Synonymy
Taxonomic synonyms occur when an organism is described multiple times under different names. As there can be only one valid name (senior synonym), the additional names applied to this species are considered junior synonyms. The name and date following the synonym indicates the author and year the species name was published. The following taxonomy and previous synonyms of the Argentine ant were described by Alexander Wild (Wild 2004).
Hypoclinea humilis Mayr 1868
Iridomyrmex humilis (Mayr); Emery 1888
Iridomyrmex rigrandensis; Borgmeier 1928
Linepithema riograndense (Borgmeier); Shattuck 1992
Linepithema humile (Mayr); Shattuck 1992
Distribution
Linepithema humile is native to the Parana river drainage basin in northern Argentina, Paraguay, Uruguay and Brazil (Wild 2004). Linepithema humile was discovered outside of its native range before 1858, in the Autonomous Region of Madeira, Portugal (Wetterer et al. 2006). Since the mid-1800s, this insect has been dispersed globally. It was likely dispersed around the globe through human trade, although the specific sources of introductions are unknown. Specimens have been found in South American countries outside of its native range, as well as in several European countries, South Africa, Australia, and the United States. Within the United States, it has been documented throughout the southeastern and southern states and up the western coastline (Wild 2004). It is considered an invasive species in all of these locations. In Florida, it was first observed in 1914 and was officially documented in 1932 (Deyrup et al. 2000).
Description
Linepithema humile is an ant in the subfamily Dolichoderinae, which is characterized by having a single connecting node, called a petiole, between the thorax and abdomen, and by their lack of both a stinger and an acidopore (an opening on some ants from where formic acid is sprayed). It can be distinguished from other Dolichoderinae ants by the pointed shape of its petiole, and by the lack of any spines on the back end of the thorax. Additionally, the gaster of this species is vertically aligned, oriented straight up and down, while others in the subfamily angle forward towards the thorax. The gaster is the posterior body region, also called the abdomen.
Linepithema humile individuals are reddish brown, occasionally darkening to black at the tip of the gaster. Workers range between 2.2 - 2.6 mm in length. The thorax (the middle body segment in insects) of this ant slopes downward to the juncture between the mesothorax and the metathorax, then ascends again, making the metathorax appear like a hill (Figure 2). These ants have a single node (petiole) between the thorax and the gaster. The petiole connects the thorax and abdomen and looks like a bump or protrusion. The petiole is erect, with a broad base that dwindles to a narrow edge (Figure 3). The antennae are elbowed, with 12- segments and no club. Linepithema humile can be differentiated from other Linepithema species by looking for large erect hairs on the thorax. The Linepithema humile thorax is comparatively hairless to others in the genus. The compound eyes of Linepithema humile are proportionally larger than other species in the genus, averaging 100 ommatidium (eye facets) per eye (Wild 2004). The head of this species is longer than it is wide. The clypeus (frontal plate on the head, directly above the jaws) of this species hosts one to three pairs of setae, located between the jaws (Figure 4) (MacGown et. al 2016; Wild 2004).

Credit: April Noble, from www.antweb.com
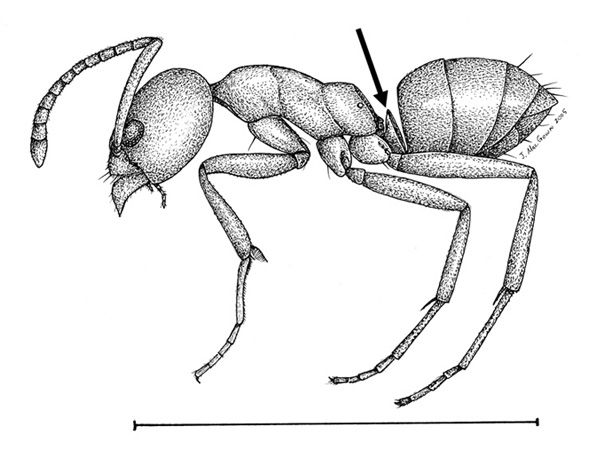
Credit: Joe. A. MacGown, Mississippi State University. From https://mississippientomologicalmuseum.org.msstate.edu/

Credit: Michele Esposito, California Academy of Sciences. From www.antweb.org
The Linepithema humile queen is larger than the workers of the species and has wings when unmated (Figure 5). After mating, a queen will remove her wings, leaving behind wing scars. A Linepithema humile queen has three ocelli (simple eyes) located at the top of her head (Figure 6).

Credit: Joe A. MacGown, Mississippi State University. From https://mississippientomologicalmuseum.org.msstate.edu/

Credit: Joe A. MacGown, Mississippi State University. From https://mississippientomologicalmuseum.org.msstate.edu/
Linepithema humile workers are all similar in size and morphology. Linepithema humile is often confused with the odorous house ant, Tapinoma sessile. These two species look superficially similar and have similar behavioral traits. A major difference between the two species is the flattened petiole that Tapinoma sessile has between the thorax and gaster (Figure 7), rather than the erect node of Linepithema humile (Fig. 3). The best way to tell the difference between the two species without a microscope is to physically crush a living worker. The scent of a crushed odorous house ant, as the name implies, is distinctive, and is often compared to rotten coconut (Layton & MacGown 2006). When a Linepithema humile worker is crushed, they emit a musty odor.

Credit: Joe A. MacGown, Mississippi State University. From https://mississippientomologicalmuseum.org.msstate.edu/
Life Cycle
Like all other ant species, Linepithema humile undergoes complete metamorphosis encompassing four life stages: egg (Figure 8), larva, pupa (Figure 9), and adult. Development time for each of the life stages is highly dependent on temperature. For example, at 21 degrees Celsius, the pupal stage averages around 25 days to develop into an adult, while at 30 degrees Celsius pupation time drops to 8 days (Abril et al. 2010). The optimal temperature for survival is 26 degrees Celsius (Abril et al. 2010). Unlike most ant species, Linepithema humile queens do not undertake nuptial (mating) flights. Instead, mating generally takes place within the birth nest, with available nestmates (Passera & Keller 1990). Males of the species will sometimes disperse from the nest. When possible, females will preferentially mate with unrelated males present in the nest. Queens that produce inbred offspring are removed as the colony head (Keller & Passera 1993). Male reproductive individuals (alates) will grow wings and will die after successfully mating. Unmated males survive, on average, 8.5 days if they do not depart the nest for the nuptial flight, and 14.1 days if they do fly (Passera & Keller 1994).

Credit: Alex Wild, University of Texas at Austin, alexanderwild.com

Credit: Alex Wild, University of Texas at Austin, alexanderwild.com
Linepithema humile is a unicolonial species, meaning it forms multiple connected nests with numerous queens. This allows Linepithema humile to form vast interconnected colonies, with little to no aggression between neighboring nests (Helanterä et al. 2009). This lack of aggression between nearby nests benefits Linepithema humile colonies, as the colonies can spend less energy on nest defense, and more energy on collecting resources. This contributes to the invasive nature of Linepithema humile colonies because they can outcompete other species, as other species colonies must spend more energy and resources on nest defense.
Economic Importance
Linepithema humile has a mutualistic relationship with honeydew-producing insects such as aphids and scales (Figure 10). Honeydew is a sugary liquid excretion produced by many sap-feeding insect pests. In exchange for the honeydew, the ants protect the plant pests from predators and parasitoids. Such sap-feeding insects can cause significant damage to the host plants by depleting nutrients and/or vectoring diseases. Linepithema humile’s protection has been shown to increase the abundance and survival rate of numerous insect pests (Brightwell & Silverman 2010; Daane et al. 2007). For example, Linepithema humile has become a key pest in orange groves where it interferes with biological control of the Asian citrus psyllid, Diaphorina citri (Kistner et al. 2016). Without arthropod predators, Diaphorina citri populations can grow unchecked.

Credit: Alex Wild, University of Texas at Austin, alexanderwild.com
Linepithema humile is also known to infest buildings, especially during rainy, cold weather (Gordon et al. 2001). Controlling this pest within a building can become costly, and frustrating and will vary by location and services provided (Klotz et al. 2008). While the ant is a nuisance in buildings, these ants pose little health risk to people, as they lack a stinger.
There is research investigating Linepithema humile as a possible biological control agent of the pine processionary moth, Thaumetopoea pityocampa, in Portugal. The presence of Linepithema humile has been linked with a reduction of tree damage by Thaumetopoea pityocampa in Portuguese pine plantations (Way et al. 1999). Regardless of the possibility of Linepithema humile as a biological control within its native range, Linepithema humile remains an invasive pest species worldwide.
Ecological Importance
In addition to its economic importance, Linepithema humile has had a major ecological impact in many ecosystems worldwide. Its ability to rapidly produce workers, due to the multiqueen structure of its colonies, often allows this species to displace and outcompete native ant species. In a 1996 study, Linepithema humile was shown to be more successful at recruiting (the ant’s ability to locate and aggregate around novel resources) to food sources than native ant species. When several ant species arrived at food stations deployed by researchers, Linepithema humile was able to fight off other species 60% of the time (Human & Gordon 1996). The long-term consequences of Linepithema humile dominating food resources can result in a reduction of native species in the area.
Native ant species play important roles in natural ecosystems. Some native ant species function as seed dispersers for native plant species. Linepithema humile has not been shown to take over the role of native ants as seed dispersers in habitats where they have displaced the native ant community (Carney et al. 2003; Gómez et al. 2003; Gómez & Oliveras 2003). Over time, the reduction in seed dispersal associated with the invasion of Linepithema humile and displacement of native ants could lead to altered plant community composition in native areas. Linepithema humile has also been shown to disrupt plant pollination by reducing insect visitations to flowers, resulting in fewer plant seeds (Blancafort & Gómez 2005).
Native ants aren’t the only arthropods that Argentine ants disrupt or displace. Linepithema humile activity has been shown to alter the composition of entire arthropod communities (Cole et al. 1992). Linepithema humile presence can also have an indirect effect on aquatic organisms via human efforts to control it. In California, pest management professionals commonly treat the perimeter of houses infested by Linepithema humile with insecticides. Water runoff collected after these treatments was shown to contain harmful levels of the insecticides (Greenberg et al. 2010 , 2017). This contaminated water can run off of paved impervious surfaces and enter storm drains, which contaminates nearby bodies of water and poses acute and chronic toxicity risks to aquatic wildlife.
Management
Cultural/MechanicalControl
Cultural control involves altering the environment and local management practices to help reduce pest numbers. Mechanical control is directly controlling a pest through physical methods like removal or exclusion. Cultural and mechanical control are often implemented together. Regarding Linepithema humile, this means removing access to food, water, and nesting sites.
In a residential setting, storing food in ant-proof containers and picking up items in a yard that may provide a safe nesting site for the ants will reduce Linepithema humile infestations. Linepithema humile are ground nesters, typically nesting thirty to forty cm below the soil surface (Heller & Gordon 2006). They may also nest underneath logs or other protective structures. Keeping the perimeter of a building clear of leaf litter, mulch and other potential nesting materials will help reduce the possibility of Argentine ants entering the structure. Additionally, repairing and sealing cracks in a building’s exterior surfaces will reduce means of entry for ants (Daughtry 2017). Keeping the interior of a building clean, monitoring indoor plants for nesting sites, properly storing food, and reducing access to water by checking plumbing for leaks will limit resources for an encroaching ant population.
In an agricultural or garden setting, altering irrigation times/frequencies to reduce standing water can reduce Argentine ant establishment. Additionally, it is recommended to place sticky traps or other barriers around plant bases to restrict ant access to honeydew producing insects. Controlling populations of honeydew producing insects like aphids, mealybugs, or scale insects that Linepithema humile feeds on will also help control the population. Importantly, this can also work the other way, where controlling Linepithema humile may help control sap-feeding pests.
Biological Control
In Linepithema humile’s native range, it is parasitized by wasps in the family, Diapriidae (Loiácono et al. 2013). In Brazil, a parasitoid fly in the genus, Pseudacteon, attacks worker ants while foraging, causing the ants to retreat underground (Orr & Seike 1998). However, applications of these insects as possible agents for classical biological control outside of the native range have not yet been developed. Solenopsis invicta (the red imported fire ant) has been shown to limit populations of Linepithema humile when present (LeBrun et al. 2007). The two aggressive species compete for similar food resources and ecological niche. However, both species are invasive and damaging to local ecosystems, so one species should not be promoted to control the other.

Credit: Alex Wild, University of Texas at Austin, alexanderwild.com
ChemicalControl
Insecticides can be necessary to achieve control of this invasive species. Liquid baits have been shown to be effective in controlling Linepithema humile (Klotz et al. 2008). Using insecticide baits is safer than insecticide sprays because they contain less insecticide, do not rely on direct contact with the insect, and provide longer control. Similarly, granular formulations of home perimeter treatments have been shown to pose reduced risk of runoff and water contamination than liquid applications (Greenberg et al. 2010). Broad- spectrum insecticide sprays may be used to control Linepithema humile but pose additional health risks and ecological hazards. Trail pheromone disruption is another novel management method currently being studied. Ants use pheromones to mark trails leading other colony members to food sources. Disrupting these trails can drastically affect a colonies’ ability to find food, and can reduce the amount of workers present in an area (Westermann et al. 2016). Both wax-based and pheromone-based sprays are being studied to test disruption effectiveness.
References
Abril, S, Oliveras, J, Gómez, C. 2010. Effect of temperature on the development and survival of the argentine ant, Linepithema humile. Journal of Insect Science 10(97), 1–13.
Blancafort, X, Gómez, C. 2005. Consequences of the Argentine ant, Linepithema humile (Mayr), invasion on pollination of Euphorbia characias (L.) (Euphorbiaceae). Acta Oecologica, 28(1), 49–55.
Brightwell, RJ, Silverman, J. 2010. Invasive Argentine ants reduce fitness of red maple via a mutualism with an endemic coccid. Biological Invasions 12(7), 2051–2057.
Carney, SE, Brooke Byerley, M, Holway, DA. 2003. Invasive Argentine ants (Linepithema humile) do not replace native ants as seed dispersers of Dendromecon rigida (Papaveraceae) in California, USA. Oecologia, 135(4), 576–582.
Cole, R, Medeiros, AC, Loope, LL, & Zuehlke, WW. 1992. Effects of the Argentine Ant on Arthropod Fauna of Hawaiian High-Elevation Shrubland. Ecological Society of America 73(4), 1313-1322
Daane, KM, Sime, KR, Fallon, J, Cooper, ML. 2007. Impacts of Argentine ants on mealybugs and their natural enemies in California’s coastal vineyards. Ecological Entomology 32(6), 583–596.
Daughtry M. 2017. The Argentine Ant. North Carolina Cooperative Extension.
Deyrup, M, Davis, L, Cover, S. 2000. Exotic ants in Florida. In Transactions of the American Entomological Society (Vol. 126, Issues 3–4, pp. 293–326).
Gómez, C, Oliveras, J. 2003. Can the Argentine ant (Linepithema humile Mayr) replace native ants in myrmecochory? Acta Oecologica 24(1), 47–53.
Gómez, C, Pons, P, Bas, JM. 2003. Effects of the Argentine ant Linepithema humile on seed dispersal and seedling emergence of Rhamnus alaternus. Ecography, 26(4), 532–538.
Gordon, DM, Moses, L, Falkovitz-Halpern, M, Wong, EH. 2001. Effect of weather on infestation of buildings by the invasive Argentine ant, Linepithema humile (Hymenoptera: Formicidae). American Midland Naturalist 146(2), 321–328.
Greenberg, L, Rust, MK, Klotz, JH, Haver, D, Kabashima, JN, Bondarenko, S, Gan, J. 2010. Impact of ant control technologies on insecticide runoff and efficacy. Pest Management Science 66(9), 980–987.
Greenberg, L, Rust, MK, Wright, SJ, Choe, DH. 2017. Argentine ant control around homes: efficacy of treatments and urban runoff. International Journal of Pest Management 63(3), 242–250.
Helanterä, H, Strassmann, JE, Carrillo, J, Queller, DC. 2009. Unicolonial ants: where do they come from, what are they and where are they going? Trends in Ecology and Evolution 24(6), 341–349.
Heller, NE, Gordon, DM. 2006. Seasonal spatial dynamics and causes of nest movement in colonies of the invasive Argentine ant (Linepithema humile). Ecological Entomology 31(5), 499–510.
Human, KG, Gordon, DM. 1996. Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia 105(3), 405–412.
Keller, L, Passera, L. 1993. Incest avoidance, fluctuating asymmetry, and the consequences of inbreeding in Iridomyrmex humilis, an ant with multiple queen colonies. Behavioral Ecology and Sociobiology 33(3), 191–199.
Kistner, E J, Melhem, N, Carpenter, E, Castillo, M, Hoddle, MS. 2016. Abiotic and biotic mortality factors affecting Asian citrus psyllid (Hemiptera: Liviidae) demographics in Southern California. Annals of the Entomological Society of America 109(6), 860–871.
Klotz, JH, Rust, MK, Field, HC, Greenberg, L, Kupfer, K. 2008. Controlling Argentine ants in residential settings (Hymenoptera: Formicidae). Sociobiology 51(3), 579–588.
Layton, B, MacGown, JA. 2006. Control of argentine ants and odorous house ants in the home. Mississippi State University Extension
LeBrun, EG, Tillberg, CV, Suarez, AV, Folgarait, PJ., Smith, CR, Holway, DA. 2007. An experimental study of competition between fire ants and Argentine ants in their native range. Ecology 88(1), 63–75.
Loiácono, MS, Margaría, CB, Aquino, DA. 2013. Diapriinae wasps (Hymenoptera: Diaprioidea: Diapriidae) associated with ants (Hymenoptera: Formicidae) in Argentina. Psyche: A Journal of Entomology 2013(320590).
MacGown, Joe A, Whitehouse, RJ. 2016. Linepithema humile (Mayr) (the Argentine Ant). Mississippi State University, Ants (Formicidae) of the Southeastern United States.
McGlynn, TP. 1999. The worldwide transfer of ants: Geographical distribution and ecological invasions. Journal of Biogeography 26(3), 535–548.
Orr, MR, Seike, SH. 1998. Parasitoids deter foraging by Argentine ants (Linepithema humile) in their native habitat in Brazil. Oecologia 117(3), 420–425.
Way, MJ, Paiva, MR, & Cammell, ME. 1999. Natural biological control of the pine processionary moth Thaumetopoea pityocampa (Den. & Schiff.) by the Argentine ant Linepithema humile (Mayr) in Portugal. Agricultural and Forest Entomology 1(1), 27–31.
Westermann, FL, Bell, VA, Suckling, DM, Lester, PJ. 2016. Synthetic pheromones as a management technique - dispensers reduce Linepithema humile activity in a commercial vineyard. Pest Management Science 72(4), 719–724.
Wetterer, J K, Espadaler, X, Wetterer, AL, Aguin-Pombo, D, & Franquinho-Aguiar, AM. 2006. Long-term impact of exotic ants on the native ants of Madeira. Ecological Entomology 31(4), 358–368.
Wild, AL. 2004. Taxonomy and distribution of the Argentine ant, Linepithema humile (Hymenoptera: Formicidae). Annals of the Entomological Society of America 97(6), 1204–1215.