The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Rice root-knot disease is caused by different Meloidogyne species (Meloidogyne graminicola, M. hainanensis, M. incognita, M. javanica, M. arenaria, M. oryzae, M. salasi, and M. tryticoryzae). However, Meloidogyne graminicola is considered the most damaging root-knot species to Asian rice cultivation due to its ability to survive under flooded soil conditions (Bridge et al., 2005; Mantelin et al., 2017). This nematode has been found in rice nurseries, rainfed upland rice and lowland rice but is also widespread in deep-water, irrigated rice production systems. Yield loss due to Meloidogyne graminicola ranges from 28%–87% depending on disease severity and cultivar (Bellafiore et al., 2015).
Distribution
Meloidogyne graminicola has been reported in upland, irrigated, lowland and deep-water rice in South and Southeast Asia. It is also found in the United States and Latin America, and was recently reported in Africa and Europe (Bridge et al., 2005; Dutta et al., 2012; Kyndt et al., 2014; Mantelin et al., 2017; Sacchi et al., 2021).
In the United States, Meloidogyne graminicola was first reported in Stuggart, Arkansas in 1934 and then in Baton Rouge, Louisiana in 1965 (Yik and Birchfield, 1979). Since then, Meloidogyne graminicola has been reported to occur in other states like Texas, Georgia, and Mississippi (MacGowan and Langdon, 1989; EPPO, 2017).
Symptoms and Plant Part Affected
Yellowing, stunting, and hook-like galls on the roots of rice plants are the characteristic symptoms caused by Meloidogyne graminicola (Figure 1). Severely infected plants flower and mature earlier than healthy plants. Distortion and crinkling along the margins of the newly emerged leaves may also indicate infection (Bridge et al., 2005; Kyndt et al., 2014).

Credit: Hung Xuan Bui, UF/IFAS
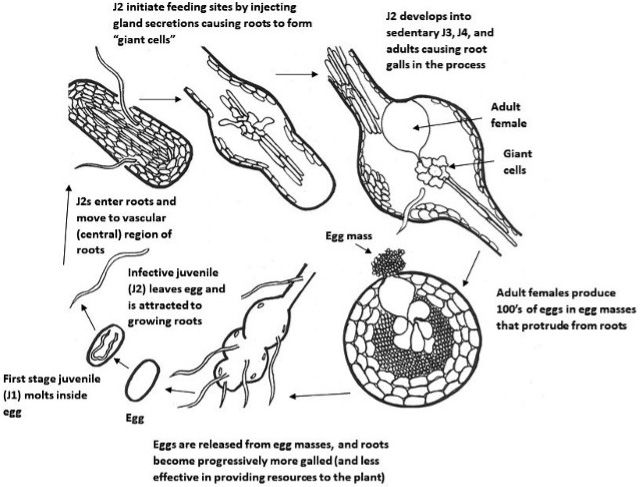
Lifecycle (Figure 2)
Meloidogyne graminicola, like other Meloidogyne species, develops from an embryo through the first and second juvenile stages within an eggshell (Figure 3). The infective second-stage juvenile (J2s) hatches and (Figure 4) infects at the elongation zone and then moves upward to the root tips where they invade the vascular cylinder to form a feeding site of three to ten cells, called a giant cell. Simultaneously, the neighboring cells start to divide to form a typical gall or root-knot. Male and female J3s become round and sedentary inside the gall and continuously molt to J4s and adults (Figure 5). Meloidogyne graminicola females commonly reproduce asexually through parthenogenesis. What makes Meloidogyne graminicola different from other Meloidogyne species is that females lay eggs typically within host tissues as an adaption to the flooded conditions found in rice fields. The newly hatched J2s (Figure 4) can stay inside the gall or move intercellularly to establish a new giant cell within the same root. In rare cases (c.a 0.5%), Meloidogyne graminicola reproduces through sexual reproduction in which adult males remodel back to a vermiform shape, while adult females keep their pear-shape. Adult males fertilize females and leave the root afterward. The life cycle of Meloidogyne graminicola can be completed in as few as 19 days under ideal environmental conditions. A single female can lay 500 to 1000 eggs during its lifetime (Bridge et al., 2005; Kyndt et al., 2014; Mantelin et al., 2017).
Credit: undefined
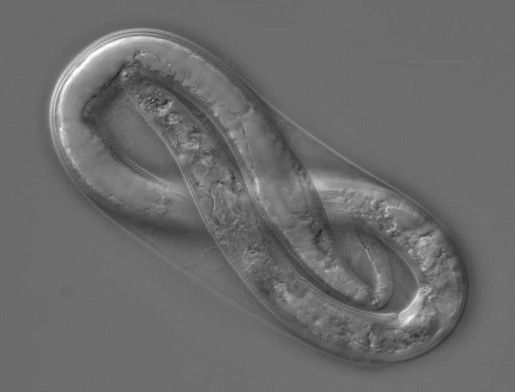
Credit: Hung Xuan Bui, UF/IFAS
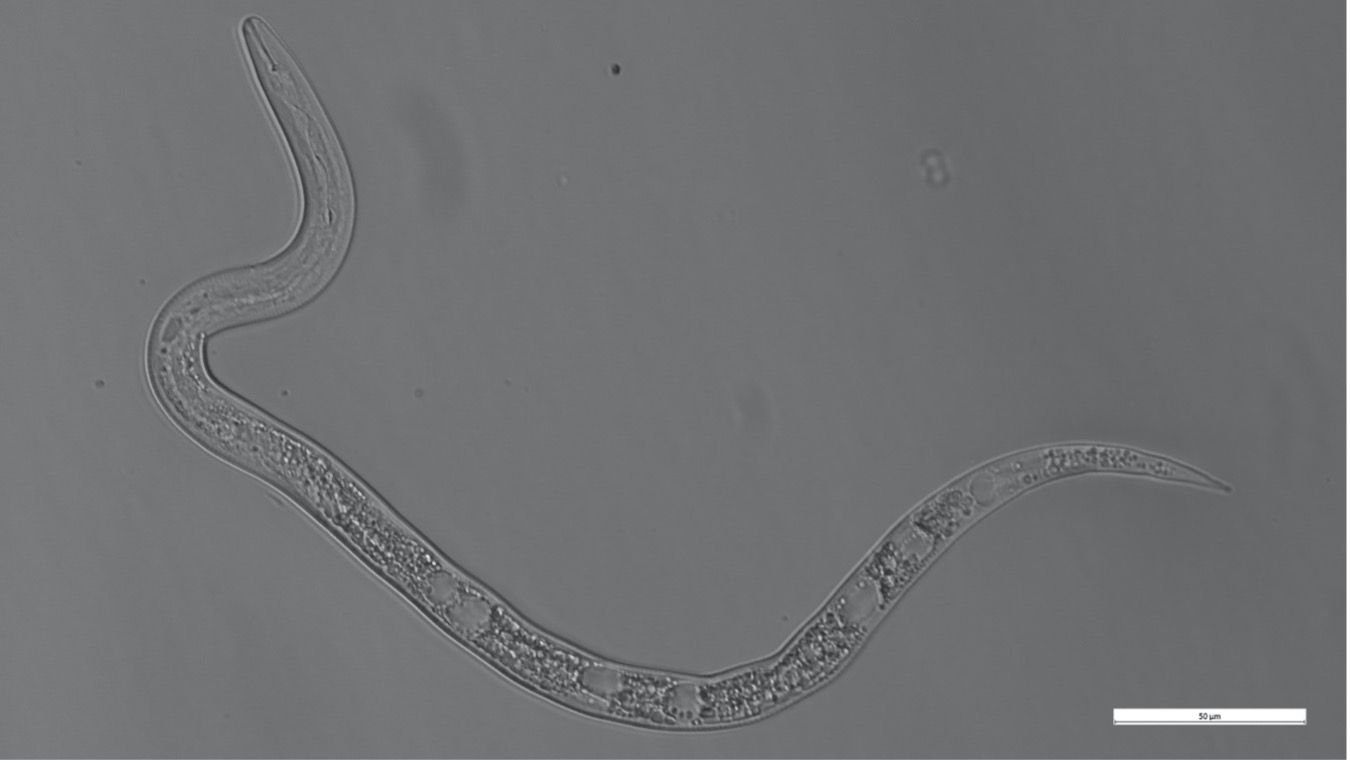
Credit: Hung Xuan Bui, UF/IFAS
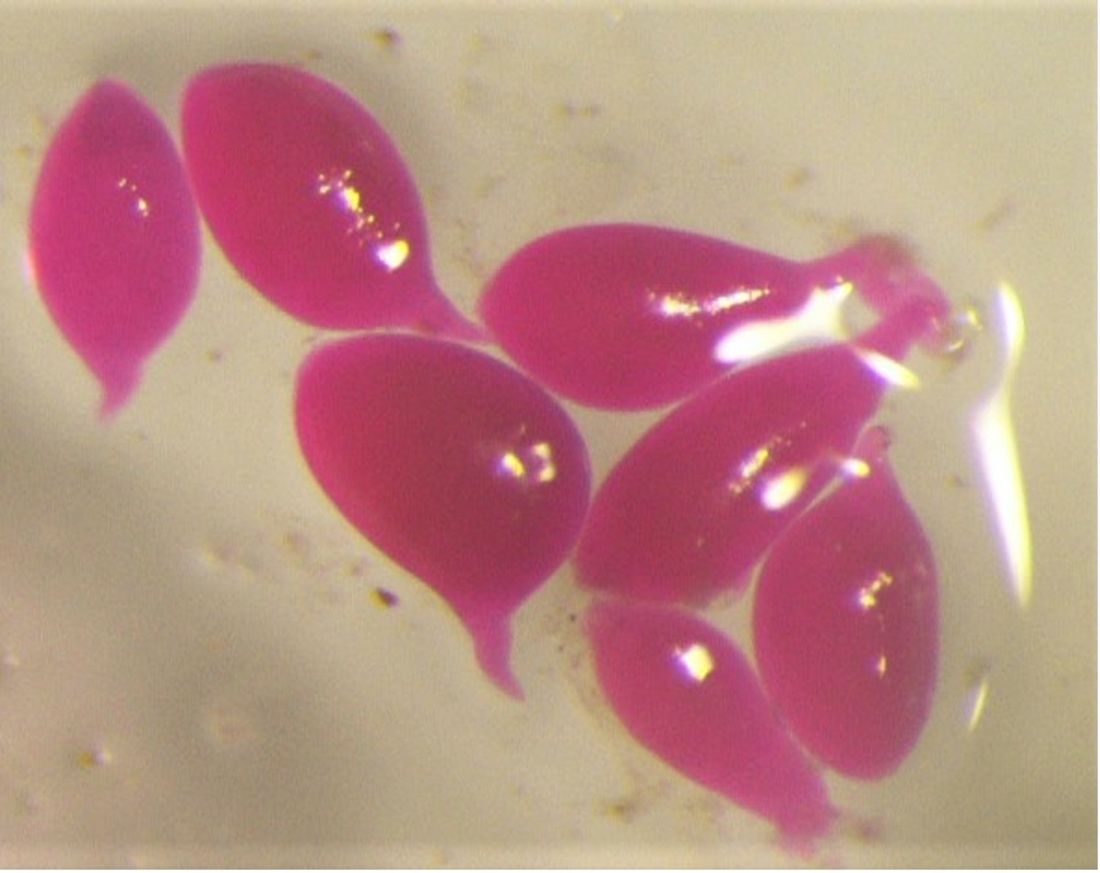
Credit: Hung Xuan Bui, UF/IFAS
Diagnosis
Meloidogyne graminicola induces the formation of characteristic hooked-like galls, normally at the root tips (Figure 1 & 7). Disease severity can be evaluated based on a root-knot galling index for rice root-knot (Figure 8). In the field, symptoms can be observed as heavily reduced growth, empty grains, reduced tillering, chlorosis, and wilting, all of which will typically occur in patches (Figure 7) (Bridge et al., 2005; Kyndt et al., 2014; Mantelin et al., 2017).
To confirm the infection on rice is due to Meloidogyne graminicola, soil and root samples should be sent to a nematology laboratory for identification. Perineal patterns of adult females are often used as a diagnostic procedure; however, this approach can lead to misidentification due to the overlapping morphological characters with other Meloidogyne species (Chen et al., 2019; Trinh et al., 2019). Molecular identification now is more rapid and accurate for root-knot nematode species identification by both species-specific primers and sequence information of conserved regions (Htay et al., 2016; Chen et al., 2019).
For instance, our nematology laboratory molecularly identified Meloidogyne graminicola collected from a field heavily infested with nutsedge in 2019 at University of Florida the Gulf Coast Research and Education Center (GCREC), Wimauma, Florida. The identification was done by using D2A/D3B primer set. DNA extraction, polymerase chain reaction, DNA sequencing, and phylogenetic analysis as described by Oliveira et al. (2019) (Figure 6). Meloidogyne graminicola is identified in sandy soil located in GCREC. The DNA sequences were deposited in the GeneBank database under accession numbers: MZ151167 and MZ151168.
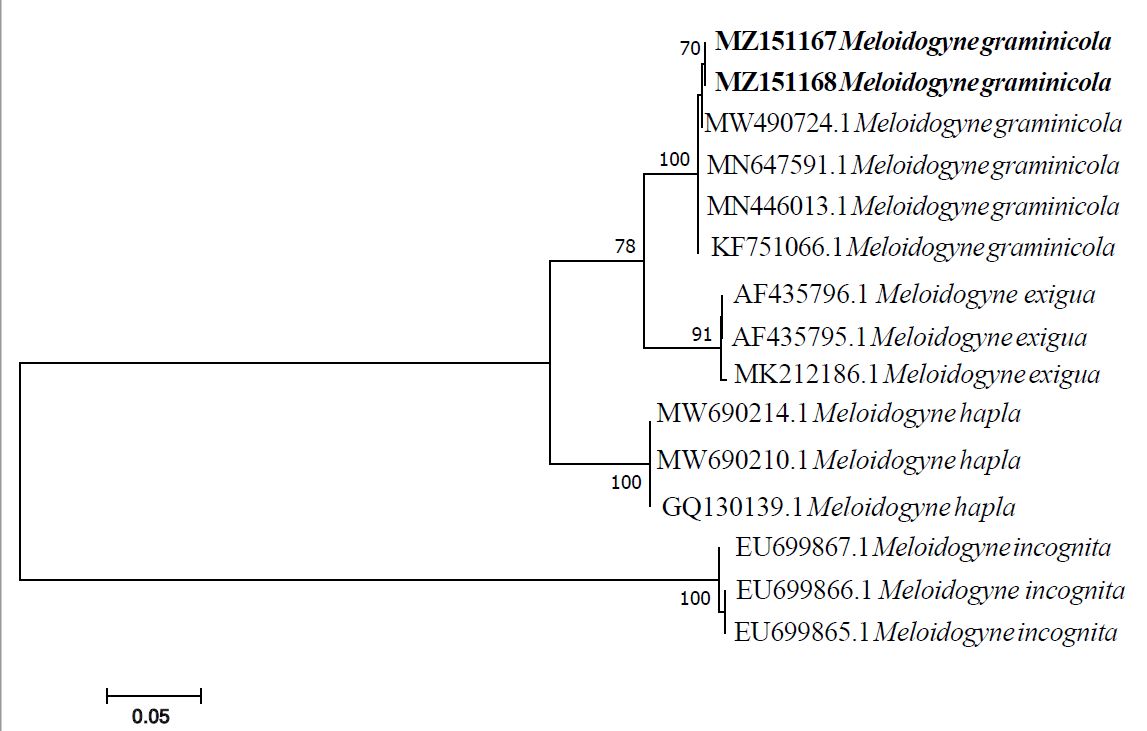
Credit: undefined

Credit: Bellafiore, PHIM Plant Health Institute, University Montpellier, IRD, CIRAD, INRAE, Institute Agro, Montpellier, France
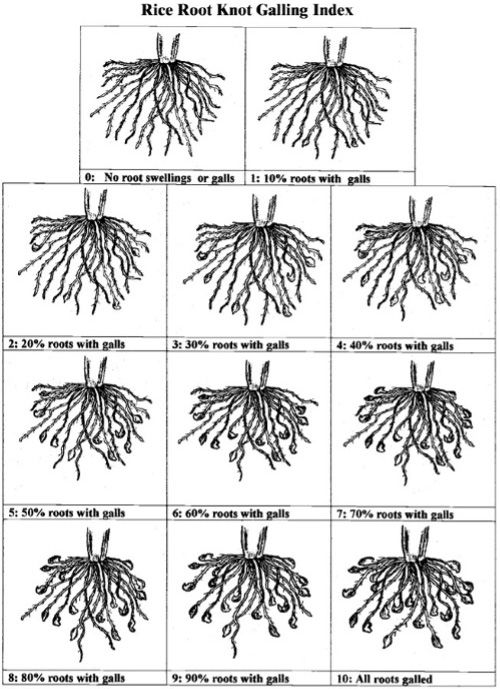
Credit: Adapted from Bridge et al. (2005)
Hosts
The main host is rice, but Meloidogyne graminicola has a very wide host range including wheat, barley, sorghum, soybean, okra, green gram, berseem, potato, onion, garlic and weed species like Cyperus rotundus, Echinochloa colonum, E. crusgalli, Leptochloa coloniculus and Phalaris minor (Bridge et al., 2005; Mantelin et al., 2017). In Florida, the nematode was found on purple nutsedge in Wimauma, FL in 2019 and on sandbur weed (Cenchrus spp.) (Handoo et al., 2003).
History and Economic Importance
In 1934, rice root-knot nematode was first reported to parasitize rice (Oryzae sativa) in Arkansas and was named Heterodera marioni, which was later renamed to Meloidogyne graminicola by Golden and Birchfield (Yik and Birchfield, 1979). Meloidogyne graminicola can cause yield loss in rice of 10 to 80% under heavy infestations. Also, its ability to adapt to deep-water conditions makes this nematode a threat to all types of rice agrosystems (Kyndt et al., 2014; Mantellin et al., 2017).
In Florida, rice is cultivated mainly in the Everglades Agricultural Area with a steady increase from 11,912 acres in 2008 to approximately 29,000 acres in 2017 (Bhadha et al., 2018). To our knowledge there have been no reports of rice root-knot nematode from the Everglades Agricultural Area.
Management
Cultural Strategies
Although Meloidogyne graminicola can survive and reproduce under flooded conditions, continuous flooding from seedling to maturity can help prevent the infection of new rice roots. Crop rotation with non-host plants such as sweet potato, cowpea, sesame, castor, sunflower, soybean, turnip, cauliflower, jute, mustard and chickpea for at least 12 months are recommended to help manage rice root-knot nematode (Dutta et al., 2012; Mantellin et al., 2017).
Host Resistance
Meloidogyne graminicola can infect most of the commercial rice cultivars, but some rice cultivars are resistant to the nematode such as wild rice Oryza longistaminata and O. glaberrima which can be used as the parents in breeding resistant varieties (Cabasan et al., 2014).
Chemical Strategies
Chemical management of Meloidogyne graminicola is common in Asia and various methods are used. Seeds can be treated with non-fumigant nematicides such as carbofuran, and seedling roots can be dipped in systemic non-fumigant chemicals such as oxamyl, phorate and carbofuran. Fumigation with chemicals such as 1, 3-dichloropropene can help to reduce the number of nematodes before planting (Dutta et al., 2012).
Other Strategies
Different biological control agents such as beneficial fungi i.e. Purpureocillium sp. Trichoderma harzianum, T. virens, Catenaria anguillulae, and beneficial bacteria i.e. Bacillus sp., Pseudomonas sp. are potential biological control organisms of Meloidogyne graminicola (Pankaj et al., 2015; Mantellin et al., 2017; Bui et al, 2020).
Selected References
Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC, Caillaud MC. 2008. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nature Biotechnology 26:909-915.
Bellafiore S, Jougla C, Chapuis É, Besnard G, Suong M, Vu, PN, De Waele D, Gantet P, Thi XN. 2015. Intraspecific variability of the facultative meiotic parthenogenetic root-knot nematode (Meloidogyne graminicola) from rice fields in Vietnam. Comptes Rendus Biologies 338: 471-483.
Bridge J, Plowright RA, Peng D. 2005. Nematode parasites of rice. Plant parasitic nematodes in subtropical and tropical agriculture 2: 87-130.
Bui HX, Hadi BA, Oliva R, Schroeder NE. 2020. Beneficial bacterial volatile compounds for the control of root-knot nematode and bacterial leaf blight on rice. Crop Protection 135: 104792. https://doi.org/10.1016/j.cropro.2019.04.016
Cabasan MT, Kumar A, Bellafiore S, De Waele D. 2014. Histopathology of the rice root-knot nematode, Meloidogyne graminicola, on Oryza sativa and O. glaberrima. Nematology 16: 73-81.
Chen JW, Chen SY, Ning XL, Shi CH, Cheng X, Xiao S, Liu GK. 2019. First report of Meloidogyne graminicola infecting Chinese Chive in China. Plant Disease 103: 11, 2967. https://doi.org/10.1094/PDIS-06-19-1241-PDN
Dutta TK, Ganguly AK, Gaur HS. 2012. Global status of rice root-knot nematode, Meloidogyne graminicola. African Journal of Microbiology Research 6: 6016-6021.
EPPO. 2017. EPPO Alert List – Meloidogyne graminicola, Rice root-knot nematode. https://www.eppo.int/ACTIVITIES/plant_quarantine/alert_list_nematodes/meloidogyne_graminicola
Handoo, ZA, Klassen, W, Abdul Baki, AA, Bryan, HH, Wang, Q. 2003. First record of rice-root nematode (Meloidogyne graminicola) in florida [abstract]. Journal of Nematology. 35: 342.
Htay C, Peng H, Huang W, Kong L, He W, Holgado R, Peng D. 2016. The development and molecular characterization of a rapid detection method for rice root-knot nematode (Meloidogyne graminicola). European Journal of Plant Pathology 146: 281-291.
Kyndt T, Fernandez D, Gheysen G. 2014. Plant-parasitic nematode infections in rice: molecular and cellular insights. Annual Review of Phytopathology 52: 135-153.
Mantelin S, Bellafiore S, Kyndt T. 2017. Meloidogyne graminicola: a major threat to rice agriculture. Molecular Plant Pathology 18: 3-15.
MacGowan, JB, and Langdon, KR. 1989. Hosts of the rice root-knot nematode Meloidogyne graminicola. Fla. Department Agriculture and Consumer Services, Division of Plant Industry. https://www.fdacs.gov/content/download/10961/file/nem172.pdf
Oliveira, CJ, Subbotin, SA, Álvarez-Ortega, S, Desaeger, J, Brito, JA, Xavier, KV, Freitas, LG, Vau, S, Inserra, RN. 2019. Morphological and molecular identification of two Florida populations of foliar nematodes (Aphelenchoides spp.) isolated from strawberry with the description of Aphelenchoides pseudogoodeyi sp. n. (Nematoda: Aphelenchoididae) and notes on their bionomics. Plant Disease 103: 2825-2842. DOI: 10.1094/PDIS-04-19-0752-RE.
Pankaj Hs, Singh K, Lal J. 2015. Management of rice root-knot nematode, Meloidogyne graminicola in rice (Oryza sativa). Indian Journal of Agricultural Sciences 85:701-704.
Sacchi S, Torrini G, Marianelli L, Mazza G, Fumagalli A, Cavagna B, Ciampitti M, Roversi PF. 2021. Control of Meloidogyne graminicola a Root-Knot Nematode Using Rice Plants as Trap Crops: Preliminary Results. Agriculture 11:37. https://doi.org/10.3390/agriculture11010037
Trinh QP, Le TM, Nguyen TD, Nguyen HT, Liebanas G, Nguyen TA. 2019. Meloidogyne daklakensis n. sp. (Nematoda: Meloidogynidae), a new root-knot nematode associated with Robusta coffee (Coffea canephora Pierre ex A. Froehner) in the Western Highlands, Vietnam. J Helminthol 93: 242-254.
Yik CP, Birchfield W. 1979. Host studies and reactions of rice cultivars to Meloidogyne graminicola. Phytopathology 69:497-499.