The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
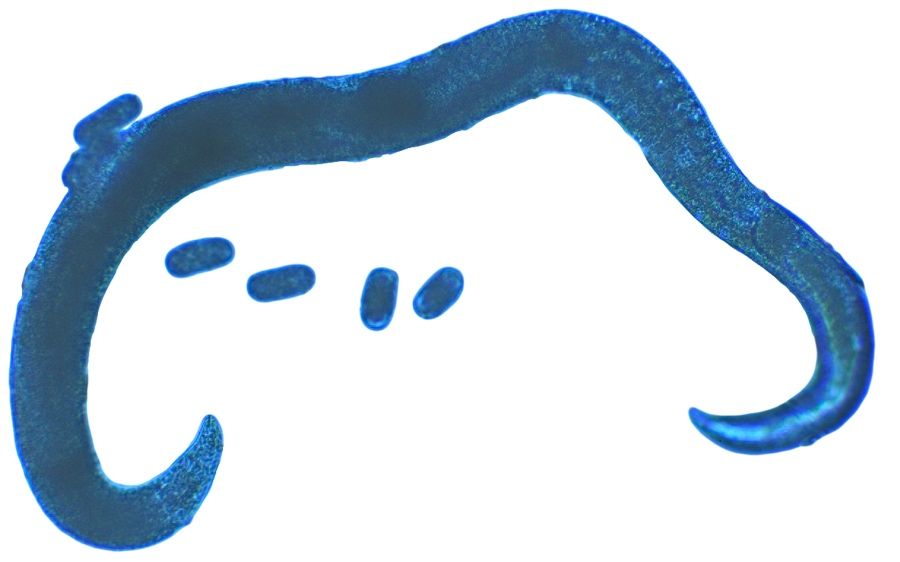
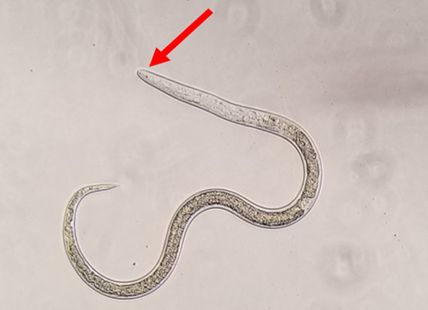
The nematode Anguina pacificae, commonly known as the Pacific shoot-gall nematode (Figure 1), is a major pathogen on annual bluegrass (Poa annua) in the Pacific region of the United States (McClure et al., 2008; Westerdahl et al., 2004). The family Anguinidae is made up of both fungal feeding nematodes and plant parasitic nematodes. Plant-parasitic genera in the Anguinidae include certain species of Anguina, Subanguina, and Ditylenchus. Unlike most other plant-parasitic nematodes, many species in this family feed on, and cause malformation of, aboveground plant parts (seeds, stems, etc.). Notable turfgrass pests in this group are the root gall nematode (Subanguina radicicola), the bentgrass nematode (Anguina agrostis) and the Pacific shoot-gall nematode Anguina pacificae. Anguina pacificae feeds on the stem of Poa annua, causing stem galls. Anguina pacificae is primarily an issue on golf courses where Poa is used as a turfgrass for putting greens, primarily in northern coastal California and Ireland. They cause chlorotic and necrotic spots that coalesce and enlarge over time, creating an inconsistent and unsightly putting surface (Figures 5 and 6).

Credit: C. L. Kammerer, University of Florida
Distribution
Anguina pacificae has a limited distribution, favoring damp, cooler climates with temperatures around 12°C to 14°C (53°F to 57°F). Anguina pacificae was first reported in northern California in close proximity to the Pacific coast (Cid Del Prado Vera and Maggenti, 1984). Subsequent surveys have found them only in golf courses in a narrow band along California’s northern Pacific coast. The first, and so far only, confirmed report of Anguina pacificae outside of North America is in Ireland (Fleming et al., 2015). It is believed that Anguina pacificae was introduced to Ireland from California on contaminated golf course equipment.
Life Cycle and Biology
The life cycle of Anguina pacificae was thoroughly documented by McClure et al. (2008), which is the source for most of the information below. The second-stage juvenile (J2) of Anguina pacificae (Figure 1) is the life stage that hatches from the egg and is the infective stage. The J2 crawl up the stem of the bluegrass plant in a film of water; this is thought to be one of the reasons this nematode is prevalent in damp regions. The J2 then enter the leaf sheath, and crawl downward within the sheath to the stem base or crown. Generally multiple nematodes enter a single crown. They enter the crown tissue and manipulate the plant cells in a way that a gall starts to form around them as they feed. Within the gall the nematodes molt into third-stage juveniles, then fourth-stage juveniles, and finally into adults. Because Anguina pacificae reproduces sexually by amphimixes, adults are either male or female and both sexes are required for reproduction to occur. While only one male and one female adult would be needed for reproduction, it has been noted that multiple adults of both sexes have been found in a single gall. After mating, the female Anguina pacificae (Figure 2) begins laying eggs, usually 1000 or more, within the gall. As the eggs hatch, the gall will contain eggs, juveniles, and adults at the same time (Figure 3). After 36 days or so post infection the females die, leaving eggs and juveniles behind. Around 80 days after infection the gall begins to rot and the J2 leave the gall to search for a new host. As the gall rots, the aboveground portion of the plant dies, causing chlorotic and later necrotic patches on the turf surface (Figure 6). When there is a lack of water, J2 are able to enter an anhydrobiotic state until sufficient moisture is present to resume nematode activity. In anhydrobiosis the nematodes can remain in a state of dormancy for up to 17 months and be readily moved on contaminated equipment.
Credit: A. Habteweld, University of Florida

Credit: C. L. Kammerer, University of Florida
Symptoms and Diagnosis
The major indicator of Anguina pacificae on an individual plant is an enlarged crown or gall (Figures 4 and 5). The most characteristic turf symptom of Anguina pacificae are yellow patches that vary in size and shape (Figure 6). These patches will eventually become brown in color (Figure 7). Over time these patches coalesce and become larger. These larger patches can culminate in an uneven putting surface caused by a loss of turf (Fleming et al., 2015).
The easiest and most accurate way to diagnose Anguina pacificae is to look for stem galls on symptomatic plant tissue, and then mechanically rupture the galls and look for the nematodes within (Figures 3 and 4). For general Anguina pacificae assessment, pre-infective J2 can be found in soil and be detected in a typical soil nematode assay. However, for more accurate population density assessment, the UF Nematode Assay Lab recommends incubating turf plugs using a modified Baermann bowl extraction method.

Credit: C. L. Kammerer, University of Florida

Credit: C. L. Kammerer, University of Florida

Credit: M. M. Mahady, Mark M. Mahady and Associates, Inc.

Credit: M. M. Mahady, Mark M. Mahady and Associates, Inc.
Hosts
The primary host to Anguina pacificae is Poa annua, although it can also infect Agrostus stolonifera to a lesser extent (McClure et al., 2008; Fleming et al., 2015).
Importance
In coastal California and parts of Ireland, this nematode is the primary nematode concern for turfgrass managers. While Anguina pacificae has a very narrow host range made up almost exclusively of Poa annua, its effect on annual bluegrass putting greens can be devastating. Where available, nematicides used to manage Anguina pacificae are expensive, but revenue losses as golfers choose to play on other courses with better greens are even more costly. Severe infestation can cause golf greens to become unplayable, leading golf courses to renovate and regrass the greens, costing millions of dollars in renovation costs and revenue losses during the renovation process. While Anguina pacificae is not known to occur in Florida, its primary host, annual bluegrass, is commonly used as a winter overseed on golf greens in this state. If Anguina pacificae became established in Florida it could potentially cause damage to overseeded golf greens under certain conditions, but not likely to the severity as in locations where annual bluegrass is the primary grass year-round.
Morphology
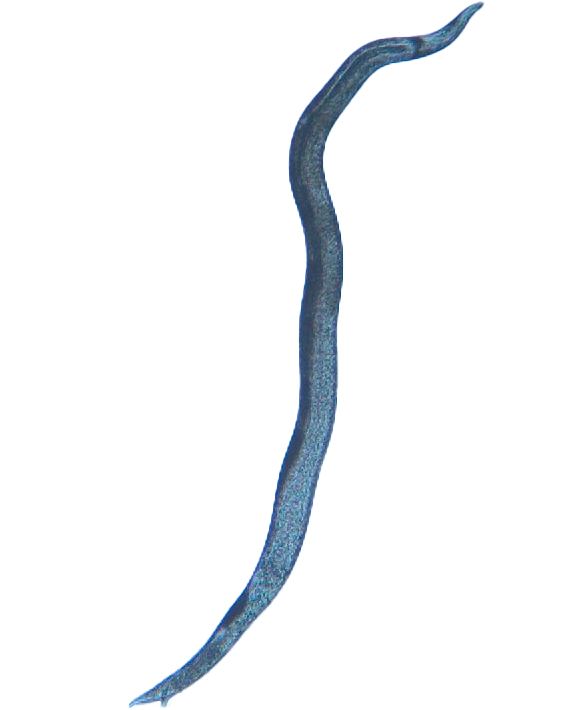
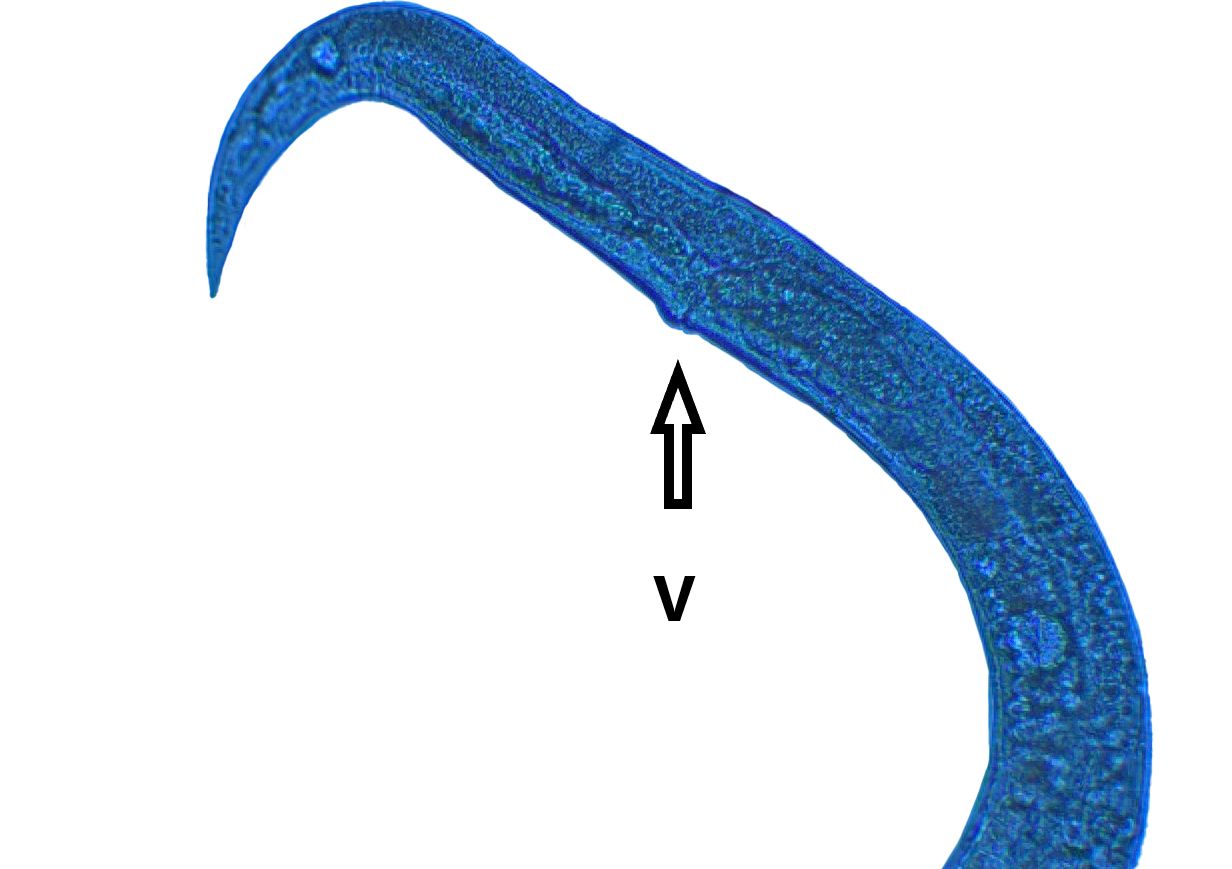
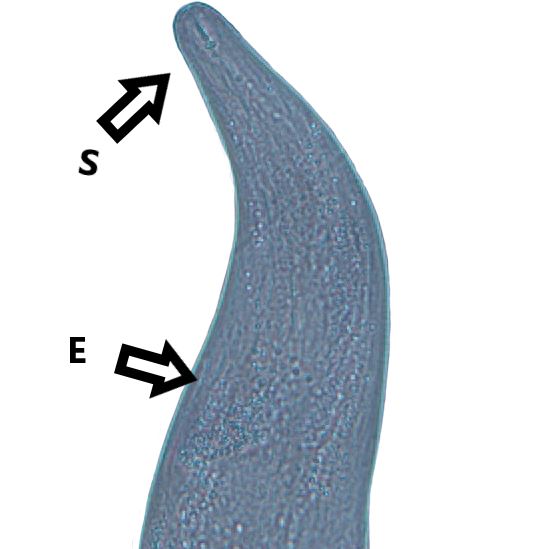
Adult Anguina pacificae are roughly 1.4-2.5 mm in length for females and 1.2-1.8 mm in length for males. The body of adults (Figure 8) is much thicker than that of the slender juveniles. A small median bulb is present behind the esophageal lumen which is connected to the feeding part of this nematode, the stylet, but is often difficult to see. Anguina pacificae is armed with a slender stylet with knobs and weakly defined cephalic framework (Figure 9). At the rear caudal end of the female, the vulva is situated close to the tail (Figure 10) (Cid Del Prado Vera and Maggenti, 1984). Annulations are present on the end of the tail (Fleming et al., 2015). These annulations are especially prominent on second stage juveniles. Second-stage juveniles recovered from soil samples are thin, have a small, weak stylet more typical of fungal-feeding than plant-parasitic nematodes, and poorly developed esophageal region (Figure 11).
Credit: undefined
Credit: A. Habteweld, University of Florida
Credit: C. L. Kammerer, University of Florida
Credit: C. L. Kammerer, University of Florida
Management
As with many other pathogens, the management of this pest includes both cultural practices as well as chemical controls. Due to this nematode’s ability to survive without moisture via anhydrobiosis, contaminated equipment poses a serious risk of spreading Anguina pacificae. Ensuring that all equipment is properly cleaned prior to use and after use is crucial. Chemical control is an effective method to reduce the low population density of Anguina pacificae and to reduce plant damage. Fluopyram (Petelewicz et al., 2020) and abamectin (Orlinski et al., 2021) have been shown to be effective for managing Anguina pacificae and reducing its damage on Poa golf greens (Figure 12). The botanical pesticide azadirachtin has shown some activity against Anguina pacificae in some trials (McClure and Schmitt, 2012), but not others (Petelewicz et al., 2020). Novel methods of controlling nematodes may also be implemented in the future. White creamy bacteria inside Anguina pacificae galls has been inhibiting the developmental cycle of the nematode (McClure et al., 2008).

Credit: M. M. Mahady, Mark M. Mahady and Associates, Inc.
Resources
Cid Del Prado Vera I, Maggenti AR. 1984. A new gall-forming species of Anguina Scopoli, 1777 (Nemata: Anguinidae) on bluegrass, Poa annua L., from the coast of California. Journal of Nematology 16(4): 386–392.
Fleming TR, Maule AG, Martin T, Hainon-McDowell M, Entwistle K, McClure MA, Fleming CC. 2015. A first report of Anguina pacificae in Ireland. Journal of Nematology 47(2): 97–104.
McClure MA, Schmitt ME, Mccullough MD. 2008. Distribution, biology and pathology of Anguina pacificae. Journal of Nematology 40(3): 226–239.
Orlinski PM, Petelewicz P, Schiavon M, Mundo‐Ocampo M, Becker JO, Baird JH. 2021. Pacific shoot‐gall disease control in annual bluegrass putting greens using a new formulation of abamectin. International Turfgrass Society Research Journal 2021: 1-10. https://doi.org/10.1002/its2.53
Petelewicz P, Orliński PM, Schiavon M, Mundo-Ocampo M, Becker JO, Baird JH. 2020. Fluopyram controls shoot-galling caused by pacific shoot-gall nematode and improves turf quality in annual bluegrass putting greens. HortTechnology 30(6): 709–718. https://doi.org/10.21273/HORTTECH04680-20
Westerdahl BB, Harivandi MA, Costello L, McCullough M, Gross P. 2004. Management of plant-parasitic nematodes on turfgrass in Northern California. Acta Horticulturae 661: 531–533. https://doi.org/10.17660/ActaHortic.2004.661.72