The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Caliothrips fasciatus (Pergande, 1895), is commonly known as bean thrips (Figure 1). Caliothrips fasciatus is in the thrips family Thripidae characterized by the presence of a down-turned ovipositor, 8-segmented antennae, and conical formation of the female's last abdominal segment (Bailey 1933). Caliothrips fasciatus was considered a serious agricultural pest in California in 1933 but soon lost its pest status. The implementation of integrated pest management, effective use of new insecticides, and resistant cultivars could be the possible reason for this decline (Hoddle et al. 2006). In Florida, Caliothrips fasciatus is commonly found in bean with low population (personal observation Khan and Seal). However, populations of Caliothrips fasciatus on bean can occasionally be damaging in Florida.

Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
Synonymy
Heliothrips fasciatus (Pergande, 1895)
Heliothrips fasciatus (Hinds, 1902)
Caliothrips woodworthi (Daniel, 1904)
Hercothrips fasciatus (Hood, 1927)
Distribution
Caliothrips fasciatus is native to North America and western Mexico (Hoddle et al. 2006). In the United States, Caliothips fasciatus has been reported from Alabama, Arizona, California, Florida, Idaho, Louisiana, Nevada, South Carolina, Texas, Washington, and Wyoming. Caliothrips fasciatus was widely distributed in urban, agricultural, and natural areas in California (Bailey 1933). The range of bean thrips also includes the west coast of Canada, Mexico, the state of Bahia in Brazil, and China (Bailey 1933, 1940, Hoddle et al. 2006, 2012, Mound et al. 2011, 2016). Caliothrips fasciatus is a quarantine pest in Australia and New Zealand (Hoddle et al. 2006). The distribution of Caliothrips fasciatus elsewhere can be considered uncertain (Mound et al. 2011).
Description
Adults of Caliothrips fasciatus are uniformly dark brown. Females are fusiform, averaging 1.1 mm in length. The antenna is 8-segmented; the first two segments are uniformly brown; the third and fourth segments are yellow with the apical half light brown and with a forked sensorium; the anterior half of the fifth segment is brown, and the remainder of segments are a uniform brown. The terminal antennal segments are very long and needle-like. The 8th segment is less than twice as long as the 7th segment. The head is not constricted at the base (Figure 2). The pronotum possesses equiangular reticles and many markings within each reticle without any long setae. The metanotum is irregularly reticulate with one pair of major setae close to the anterior margin (Figure 3) (Bailey 1933).

Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
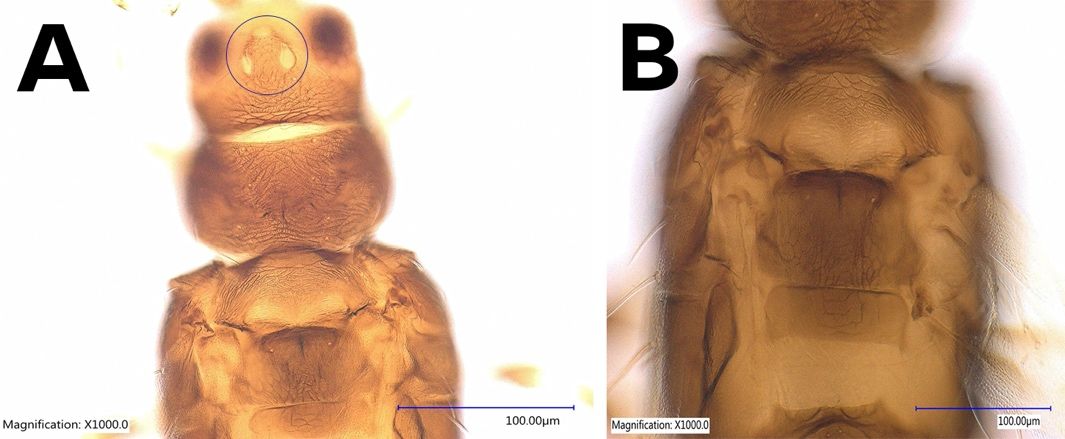
Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
Both sexes of Caliothrips facsiatus are macropterous (having long wings). The fore wing is grayish brown with two white cross bands at the base and sub-apically. The second vein in the forewing possesses five to seven setae, and the costal cilia are shorter than the costal setae. The setal row of the first vein in the forewing is incomplete and uniformly spaced. The setal row of the second vein in the forewing is complete, close together but uniformly spaced. The forewing postero-margin cilia is undulated near the apex. Hind wings are uniformly grayish brown, with one longitudinal vein in the center of the wing that becomes indistinct near the base (Figure 4) (Bailey 1933).

Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
Femora is dark brown and light-colored at the tip. Central portion of tibia is dark brown with yellow extremities. Tarsi are elongated and 1-segmented. Tergites reticulate on lateral thirds; reticles are longer than wide with numerous internal markings. The 8th abdominal tergite is with posteromarginal craspedum medially but the comb of microtrichia laterally. The median split on the 10th segment is about half as long as tergite (Figures 5 and 6). The third to seven male sternites are with small oval glandular area or pore plate and tergite nine with three pairs of stout setae medially, the median pair thorn-like. The male sternites 3-7 are with a small transverse pore plate (Figure 7) (Bailey 1933).

Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department
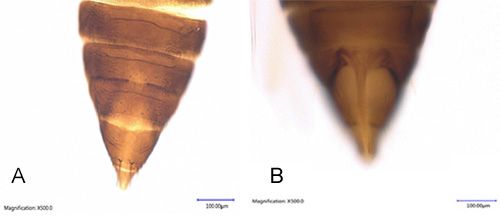
Credit: Rafia A. Khan, UF/IFAS Entomology and Nematology Department

Credit: Roshan Adhikari, UF/IFAS Entomology and Nematology Department

Credit: Roshan Adhikari, UF/IFAS Entomology and Nematology Department
Life Cycle
The detailed life cycle parameters of Caliothrips fasciatus were studied by Bailey (1933) in California on pears and beans. Caliothrips fasciatus reproduces bisexually and parthenogenetically (arrhenotoky, a form of parthenogenesis in which the unfertilized eggs develop into males). Females are produced from fertilized eggs, and males from unfertilized eggs. The sex ratio of Caliothrips fasciatus is 2:1 (female:male). In California, Bailey (1933) found that the overwintering adults migrated to prickly lettuce (Lactuca serriola L.) and sow thistle (Sonchus spp. L.) of the plant family Asteraceae and completed two generations from April to early June. The generation time of Caliothrips fasciatus is three weeks during summer, and it can complete six or seven generations from April to October. When natural vegetation senesces, adults migrate to crops in late June or early July. Females insert eggs within plant tissue in the early morning and late afternoon. There are two larval instars of Caliothrips fasciatus, found primarily on the undersides of leaves. Mature second instars drop from the host to the ground for pupation, passing through prepupal and pupal stages. Eggs hatch in about seven days; the first and second instars feed for ten days, and the pupal stage is five days long during the summer in central California. Caliothips fasciatus adults are active between 50oF (10ºC) and 117oF (47ºC) while the optimum activity ranges from 75ºF (24ºC) and 90ºF (32ºC). In California, a 60% natural mortality of Caliothrips fasciatus was reported. The adult longevity of Caliothrips fasciatus varies by season, averaging 22 days on pears (Pyrus spp. plant family Rosaceae) and beans (Phaseolus vulgaris L. plant family Fabaceae). Caliothrips fasciatus adults disperse into crop fields in irregular patterns with dispersion depending on wind and location of host plants. Females require mating with males to produce female offspring. Females, once mated, lived 20 days (Bailey 1933).
Hosts
Table 1. Caliothrips fasciatus exhibits a wide range of hosts listed below.
Economic Importance
Both larval and adult Caliothrips fasciatus cause direct feeding injury to plants by using their asymmetrical piercing-sucking mouthparts. They consume the plant sap from the epidermal and mesophyll cells of the host leaves (Lewis 1973, Hunter and Ullman 1992). However, large aggregations of larvae cause more injury to host plants than adults. Larvae and adults feed on foliage, flowers, fruits, and stems of the host plant (Childers and Achor 1995). Feeding injury of Caliothrips fasciatus appears as silvering or bronzing on leaves leading to defoliation and plant death. In pears, feeding injury of Caliothrips fasciatus results in “sun-scald” on immature fruits and reduces the productivity of the tree in the following growing season. Caliothrips fasciatus can feed on immature fruits producing scarring and downgrading at the packinghouse. Caliothrips fasciatus may also cause cosmetic injury on fruit surfaces from black fecal droplets (Russell 1912, Bailey 1933). Caliothrips fasciatus was identified as a quarantine pest of several shipments of navel oranges sent from California to Australia in the 1996-1997 season. California growers were concerned about the potential loss of the Australian citrus market, however, Caliothrips fasciatusdid not develop into a serious pest of California citrus (Hoddle et al. 2006).
Management
Monitoring
Green sticky cards are more effective at capturing Caliothrips fasciatus than yellow, white, or blue sticky cards, but all colors can be used to monitor population trends of Caliothrips fasciatus (Harman et al. 2007). Caliothrips fasciatus is commonly observed on bean plants in Florida. The larvae are more abundant on the underside of the leaves than the upper side and adults are more common in flowers than leaves (personal observation Khan and Seal 2019).
Chemical Control
Insecticides of different modes of action can be effective against Caliothrips fasciatus when applied to foliage (Greenberg et al. 2009, Renkema et al. 2020). However, information on registered insecticides to manage Caliothrips fasciatus is scarce in the literature. Leesch et al. (2007) reported that the combination treatment of ozone, vacuum, and carbon dioxide could control the overwintering Caliothrips fasciatus on navel oranges shipped to Australia without damaging the fruits. Fumigation of California navel oranges with ethyl formate mixed with carbon dioxide effectively managed adult Caliothrips fasciatus without affecting fruit quality (Bikoba et al. 2019).
Biological Control
Biological control agents of thrips can be useful to manage Caliothrips fasciatus populations. Important predators of thrips include minute pirate bug Orius spp. Wolff, Anthocoris sp. Fallen (Hemiptera: Anthocoridae); ladybird beetles Hippodamia spp. Dejean (Coleoptera: Coccinellidae); syrphid larvae such as Syrphyus sp. Fabricius and Sphaecrophoria sp. Thomson (Diptera: Syrphidae); lacewing Chrysopa spp. Leach (Neuroptera:Chrysopidae), predaceous thrips Aeolothrips spp. Priesner (Thysanoptera: Aeolothripidae), and Scolothrips sp. Hinds (Thysanoptera: Thripidae). Both adults and nymphs of Orius insidious var. tristicolor White, larva of Chrysopa californica Coq., larva of Hippodamia convergens Guerin, larva of Aeolothrips kuwanai Moulton, larva of A. fasciatus L., mites Phytoseiulus macropilis Banks (Acari: Phytoseiidae) and Anystis agilis von Heyden (Trombidiformes:Anystidae) were found to predate on larval Caliothrips fasciatus in central California (Bailey 1933). The predatory thrips such as Aeolothrips spp., Franklinothips vespiformis Craw. (Thysanoptera: Aeolothripidae), Franklinothrips orizabensis Back (Thysanoptera: Aeolothripidae), and Scolothrips sexmaculatus Pergande (Thysanoptera: Thripidae) are potential predators of Caliothrips fasciatus (Dreistadt et al. 2004). The hymenopteran parasitoid Thripoctenus russelli Craw. (Hymenoptera: Eulophidae) was recognized as the most important natural enemy of Caliothrips fasciatus in California (Bailey 1933).
Cultural Control
Heavy rainfall can suppress Caliothrips fasciatus populations (Greenberg et al. 2009) and overhead irrigation would be useful for management. Caliothrips fasciatus can complete its life cycles on many cultivated crops and wild plants. Therefore, removal of weeds from and around the field may help reduce Caliothrips fasciatus in the crop and the migration to the crop (Parker et al. 2013).
Selected References
Bailey S. 1933. The biology of the bean thrips. Hilgardia 7(12): 467-522. https://doi.org/10.3733/hilg.v07n12p467
Bailey SF. 1937. The bean thrips. Monograph Bulletin 609. University of California Experiment Station, Berkeley, CA, USA.
Bailey SF. 1937. Thrips of economic importance in California. Circular of the University of California Berkeley Agricultural Experiment Station 346, Berkeley, CA, USA.
Bailey SF. 1940. The distribution of injurious thrips in the United States. Journal of Economic Entomology 33(1): 133-136. https://doi.org/10.1093/jee/33.1.133
Bikoba VN, Pupin F, Biasi WV, Rutaganira FU, Mitcham EJ. 2019. Use of Ethyl Formate fumigation to control adult bean thrips in navel oranges. Journal of Economic Entomology 112(2): 591-596. https://doi.org/10.1093/jee/toy369
Carrillo CIC, Funderburk J, Snyder WE. 2016. Thrips (Thysanoptera) collected from Solanum dulcamara (Solanales: Solanaceae) in Washington and Idaho. Florida Entomologist 99(2): 306-307. https://doi.org/10.1653/024.099.0225
Childers CC, Achor DS. 1995. Thrips feeding and oviposition injuries to economic plants, subsequent damage and host responses to infestation. In Thrips biology and management pp. 31-51. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-1409-5_3
Dreistadt SH, Clark JK, Flint ML. 2004. Pests of landscape trees and shrubs: an integrated pest management guide, third ed. University of California Agricultural and Natural Resources Publication 3359, Oakland, Ca, USA.
Greenberg SM, Liu TX, Adamczyk JJ. 2009. Thrips (Thysanoptera: Thripidae) on cotton in the Lower Rio Grande Valley of Texas: Species composition, seasonal abundance, damage, and control. Southwestern Entomologist 34(4): 417-430. https://doi.org/10.3958/059.034.0406
Harman JA, Mao CX, Morse JG. 2007. Selection of color of sticky trap for monitoring adult bean thrips, Caliothrips fasciatus (Thysanoptera: Thripidae). Pest Management Science 63(2): 210-216. https://doi.org/10.1002/ps.1322
Hoddle MS, Stosic CD, Mound LA. 2006. Populations of North American bean thrips, Caliothrips fasciatus (Pergande) (Thysanoptera: Thripidae: Panchaetothripinae) not detected in Australia. Australian Journal of Entomology 45(2): 122-129. https://doi.org/10.1111/j.1440-6055.2006.00528.x
Hoddle MS, Mound LA, Paris DL. 2012. Thrips of California. CBIT Publishing, Queensland. Browse species | Thrips of California (lucidcentral.org)
Hunter WB, Ullman DE. 1992. Anatomy and ultrastructure of the piercing-sucking mouthparts and paraglossal sensilla of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). International Journal of Insect Morphology and Embryology 21(1): 17-35. https://doi.org/10.1016/0020-7322(92)90003-6
Leesch JG, Tebbets JS, Tebbets JC. 2004, August. Using ozone for controlling bean thrips in the navels of oranges being exported to Australia. In Controlled Atmosphere and Fumigation in Stored Products International Conference pp. 8-13.
Lewis T. 1973. Thrips, their biology, ecology and economic importance. London, Academic Press.
Mound LA, Zhang HR, Bei YW. 2011. Caliothrips tongi sp. n. (Thysanoptera: Thripidae) from China and a dubious record of North American bean thrips. Zootaxa 2736: 57-62. https://doi.org/10.11646/zootaxa.2736.1.5
Mound LA, Nakahara S, Tsuda DM. 2016. Thysanoptera-Terebrantia of the Hawaiian Islands: an identification manual. ZooKeys 549: 71-126. https://doi.org/10.3897/zookeys.549.6889
Parker BL, Skinner M, Lewis T. 2013. Thrips biology and management (Vol. 276). Springer Science & Business Media.
Pergande T. 1985. Observations on certain Thripidae. Insect life 7 pp.390-395.
Pinent SM, Nondillo A, Botton M, Redaelli LR, Pinent CEDC. 2011. Species of thrips (Insecta, Thysanoptera) in two strawberry production systems in Rio Grande do Sul State, Brazil. Revista Brasileira de Entomologia 55(3): 419-423. https://doi.org/10.1590/S0085-56262011005000032
Renkema JM, Krey K, Devkota S, Liburd OE, Funderburk J. 2020. Efficacy of insecticides for season-long control of thrips (Thysanoptera: Thripidae) in winter strawberries in Florida. Journal of Crop Protection 127: 104945. https://doi.org/10.1016/j.cropro.2019.104945
Russell HM. 1912. The bean thrips (Heliothrips fasciatus Pergande). Bulletin, USDA, Bureau of Entomology 118:1-49. https://doi.org/10.5962/bhl.title.63404