The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Toxorhynchites rutilus is one of the largest known species of mosquitoes in North America. Larvae of Toxorhynchites are aquatic predators and prey upon aquatic invertebrates with preference under some circumstances for larvae of other mosquitoes, a characteristic that can contribute to the use of Toxorhynchites mosquitoes as potential biological controls against container-inhabiting mosquitoes (Focks 2007). Larvae of Toxorhynchites can develop in natural containers (e.g., tree holes, plant leaf bases, bamboo, broken stems and internodes) and in artificial man-made containers (e.g., cans, flowerpot saucers, planters, and discarded tires) (Steffan and Evenhuis 1981). Adult females of Toxorhynchites are autogenous and feed only on carbohydrate-rich sources (e.g., nectar, honeydew, and fruit), but not blood which makes them harmless in terms of serving as a vector of pathogens to humans and animals.
Synonymy
Megarhinus rutilus Coquillett 1896
Distribution
Toxorhynchites rutilus has a wide geographical distribution in the eastern half of the United States. There are two subspecies, Toxorhynchites rutilus rutilus and Toxorhynchites rutilus septentrionalis, where the latter has a wider geographic range (Burkett-Cadena 2013) (Figure 1).
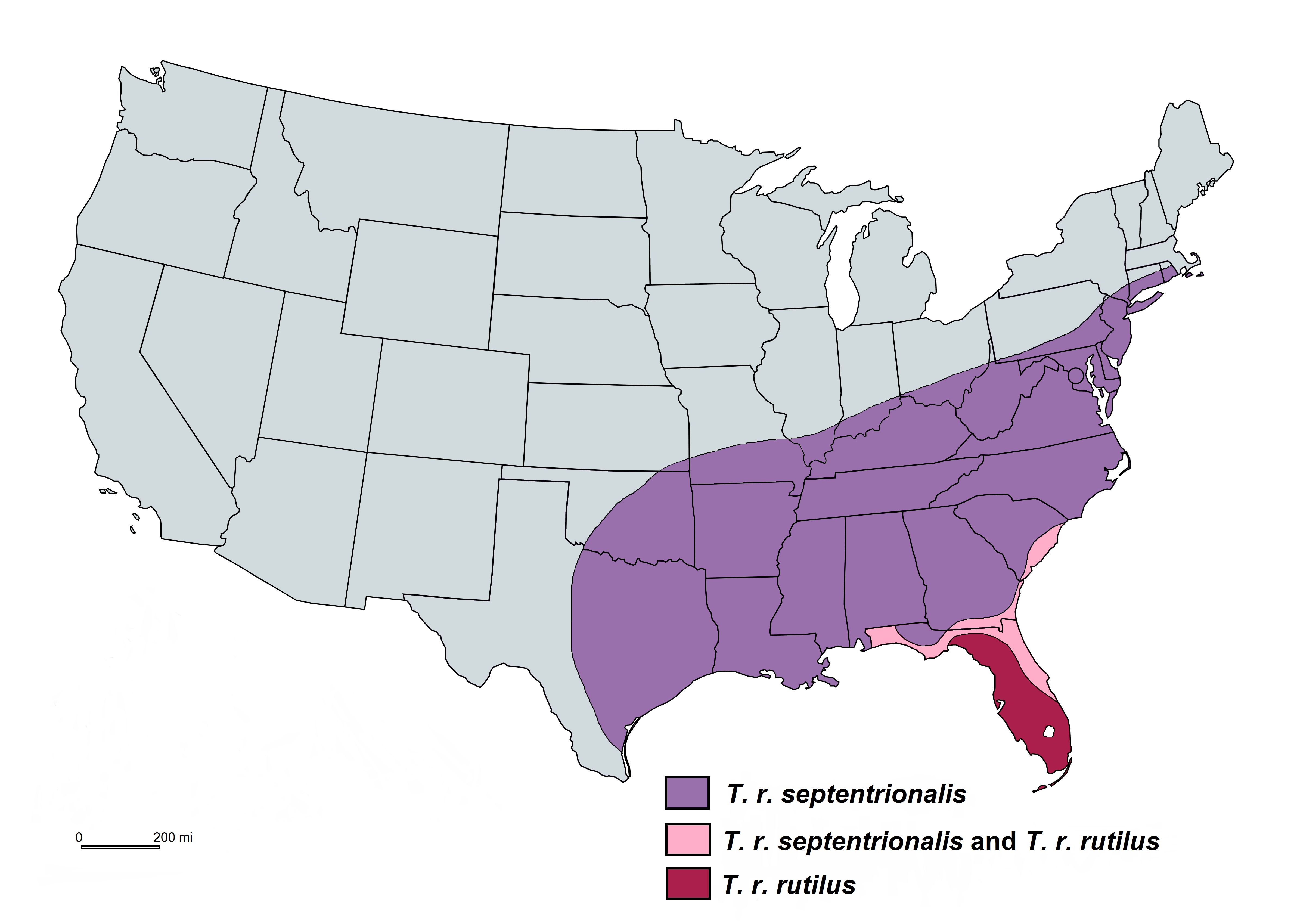
Credit: Abdullah A. Alomar, UF/IFAS
Description
Adult
The adults of Toxorhynchites are larger than most other mosquito species (Wilkerson et al. 2021) (Figure 2). The adult body consists of three parts, head (antennae, eyes, proboscis, and palps), thorax (wings and legs), and abdomen (genitalia). The adult proboscis is very long and distinctively downward curved at a 90-degree angle or more (Figure 3). The subspecies can be distinguished based on morphological differences among adult males. Male of Toxorhynchites rutilus rutilus has a pale band on the foreleg tarsus, whereas male of Toxorhynchites rutilus septentrionalis has entirely dark scaled tarsi of the forelegs (Burkett-Cadena 2013). The thorax and the entire body are covered dorsally with iridescent scales of various colors, including blue, white, green, and purple (Burkett-Cadena 2013). The scutellum is evenly rounded and has no setae (Wilkerson et al. 2021).

Credit: Nathan D. Burkett-Cadena, UF/IFAS
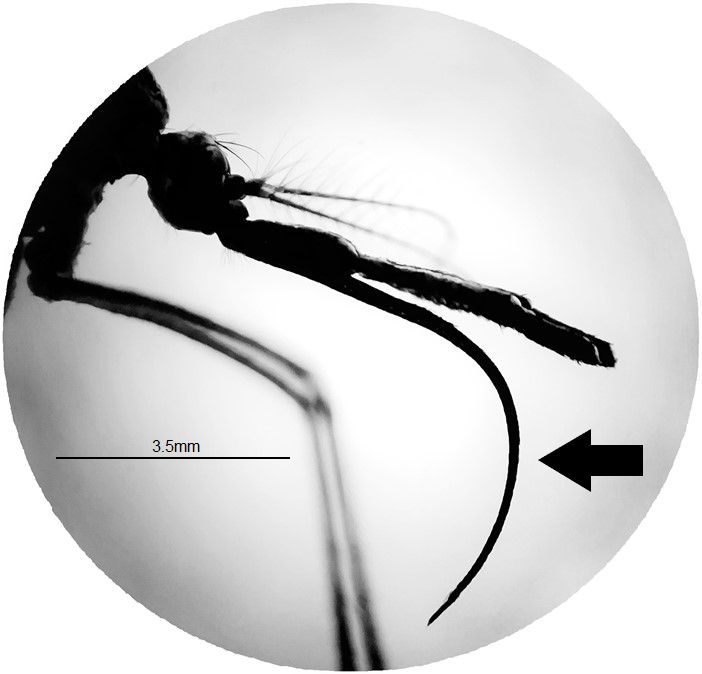
Credit: Abdullah A. Alomar, UF/IFAS
Egg
Toxorhynchites eggs are creamy white in color, oval in shape (similar to a rugby ball, an elongated ellipsoidal shape), and covered with papilliform ornamentation (Figure 4). Females deposit their eggs individually on the surface of water while in flight (hovering). The surface of the eggs is covered entirely with outer chorionic tubercles, except a small area around the micropyle (Linley 1989). The eggs are hydrophobic which allows them to float horizontally and high on top of the water surface until the emergence of first instar larvae (Steffan and Evenhuis 1981). The hydrophobic surface of the egg enables freedom of movement upon disturbance, which may be an adaptation in response to predation. The incubation period of Toxorhynchites eggs ranges from 40 to 60 hours and it is dependent on the temperature (Collins and Blackwell 2000). Since the eggs are not resistant to desiccation, they must remain in contact with water to remain viable (Steffan and Evenhuis 1981).

Credit: Abdullah A. Alomar, UF/IFAS
Larva
Larvae of Toxorhynchites are larger in size and darker in color than most other mosquito species (Figure 5 and 6). While the dorsal view of the body is dark red, the ventral view of the body is pale grayish white (Burkett-Cadena 2013). The head of larvae are square and contain a pair of compound eyes, mouthparts, and antennae. Toxorhynchites larvae are predators and prey upon other aquatic animals, including other mosquito larvae. The heavily chitinized mouthparts of Toxorhynchites larvae have comb-like mandibles which are used to grasp and consume aquatic prey. The antennae are shorter than the length of head. The thorax contains many stout spines (Burkett-Cadena 2013). The abdomen has eight apparent segments. Comb scales are absent. The siphon is short and stout with no pecten (Burkett-Cadena 2013). The anal papillae are extremely short.

Credit: Nathan D. Burkett-Cadena, UF/IFAS

Credit: Abdullah A. Alomar, UF/IFAS
Pupa
Toxorhynchites pupae have a comma-like shape, which is larger than most other mosquito pupae (Figure 7). The body of the pupa consists of head and thorax, which are fused in a cephalothorax, and abdomen. The pupa is a non-feeding, developmental stage. The cephalothorax has a pair of respiratory trumpets located on its dorsal surface, where these trumpets are used to obtain oxygen directly from the air. The abdomen has eight segments with a pair of paddles at the abdomen apex, using for swimming. Newly pupated individuals are light brown and become dark brown to black before adult emergence. The development of pupal stage lasts from 3 to 7 days, but others have observed development times as long as 23 days (Steffan and Evenhuis 1981).

Credit: Nathan D. Burkett-Cadena, UF/IFAS
Life History
Toxorhynchites undergoes complete metamorphosis (egg, larva, pupa, and adult). Unlike many mosquito species, Toxorhynchites have been observed to have a unique oviposition behavior, where gravid female exhibit counterclockwise flying for a series of ellipses above the oviposition containers prior to eject an egg during the last ellipse of downward flight (Linley 1987). This aerial mode of oviposition behavior provides females of Toxorhynchites with several advantages, including the ability to deposit their eggs in containers with limited access (e.g., small or obstructed opening) as well as reducing the possibility of females to be captured by predators that are commonly found around mosquito habitats (e.g., spiders) (Steffan and Evenhuis 1981). A female may distribute eggs over several container habitats. The continuous production of mature follicles allows females to continue laying eggs over many days (1.0-3.2 eggs per day with 58.5 eggs total during the adult lifespan, Focks et al. 1977, Focks and Boston 1979). Common oviposition sites include artificial (e.g., flowerpots, discarded tires, earthen pots, and barrels) and natural (e.g., tree hole and hollow internodes of bamboo) container habitats (Steffan and Evenhuis 1981). All larval instars are predaceous on aquatic animals, generally of their same size or smaller (Steffan 2007). A single larva may consume thousands of prey items during its development (Steffan and Evenhuis 1981, Focks 2007). The predatory behavior is considered opportunistic (ambush predator), and larvae rely on mechanoreceptors to detect prey movement passing by (Sato 1961).
Cannibalism among Toxorhynchites larvae is not uncommon, especially when alternative prey are unavailable, which depends on prey availability and the size of prey relative to larvae of Toxorhynchites (Steffan and Evenhuis 1981). Fourth instar larvae of Toxorhynchites exhibit prepupal compulsive killing behavior, where the larvae kill the prey without consumption (Lounibos 1979, Steffan and Evenhuis 1981). It is likely that prepupal killing evolved in response to the vulnerability of the pupal stage to mortality from predators, including other Toxorhynchites larvae (Collins and Blackwell 2000). The development time of Toxorhynchites larvae are dependent on several factors, such as prey density, prey size, container size, and water temperature (Lounibos 1979, Collins and Blackwell 2000). Density of prey, a measure of availability of nutrition, can strongly affect the development time of Toxorhynchites larvae (i.e., increased number of prey consumed daily accelerates development). At the end of last instar of larvae (fourth instar), larvae can molt to pupae and these pupae remain on the water surface for about 3-7 days, after which adults emerge from pupal case. Adults of Toxorhynchites are active during the daytime (Wilkerson et al. 2021). Both males and females of Toxorhynchites feed on nectar and other plant exudates with no need for blood. The females of Toxorhynchites are autogenous and obtain all nutritional requirements for egg development in adulthood from the consumption of prey during larval stages (Focks 2007).
Photoperiodic control of fourth instar diapause
Throughout its range, short daylengths experienced by early instar larvae of this species lead to diapause in the fourth instar (Bradshaw and Holzapfel 1975, Trimble and Smith 1979). Some Toxorhynchites rutilus from south Florida also diapause, which condition leads to reduced prey consumption rates (Lounibos et al. 1998). The composite index of performance (r’) for diapausing cohorts was ½ to 2/3 that of non-diapausers (Lounibos et al. 1998).
Top predator in Florida treehole community
Bradshaw and Holzapfel (1983) concluded from a year-long study of natural treeholes in northern Florida that predation by Toxorhynchites rutilus reduced abundances of culicid prey and thereby minimized larval competition among mosquito prey. The same authors also concluded that Toxorhynchites rutilus larvae preferentially occupied drought-resistant treeholes with a subcommunity that included predator-resistant mosquito prey, such as Orthopodomyia signifera (Bradshaw and Holzapfel 1988).
Natural prey of Toxorhynchites rutilus larvae in south Florida treeholes and tires
Campos and Lounibos (2000a) dissected wild-caught larvae of Toxorhynchites rutilus to determine prey types ingested from exoskeletal remains in midguts. Twenty taxa of aquatic prey were recognized, as well as remains of terrestrial arthropods of nine insect orders and mites. Mosquitoes accounted for only 6% of prey items from treeholes and 5% from tires. Prey choice was estimated, and it was high for Aedes albopictus but low for Orthopodomyia signifera and Toxorhynchites rutilus.
Life tables of Toxorhynchites rutilus aquatic stages in south Florida treeholes and tires
A companion paper by Campos and Lounibos (2000b) examined stage-specific survivorships of Toxorhynchites rutilus calculated from repeated censuses of treeholes and water-holding tires at the Florida Medical Entomology Laboratory. Survivorship from egg to adult ranged from 1.8-5.6% and varied significantly between seasons and container types. Cannibalism was imputed to be a factor influencing high mortalities in the egg and fourth instar larval stages.
Long term dynamics of Toxorhynchites rutilus and prey reduction in south Florida treeholes
The aquatic contents of ten treeholes were censused fortnightly between 1978-93 to examine the population dynamics of aquatic culicid occupants (Lounibos et al. 1997). Time series analyses showed that the presence of Toxorhynchites rutilus fourth instar larvae significantly reduced the abundance of late-stage larvae of eastern treehole mosquito Aedes triseriatus, with pupae of this prey species depleted relatively more than fourth instar larvae. Overall, this predator-prey pair was temporally decoupled, and Toxorhynchites rutilus disappeared from censuses during a 30-month drought.
Use in Biological Control
Adults of Toxorhynchites do not consume blood and so they are incapable of transmitting pathogens to humans or animals by bite. The larvae of Toxorhynchites are voracious predators and have ability to consume thousands of larvae of mosquito vectors. Additionally, late instar larvae of Toxorhynchites show prepupal compulsive killing behavior against prey larvae. Toxorhynchites larvae have ability to resist starvation and survive weeks without prey, especially at late instars (Focks 2007). Adults of Toxorhynchites has ability to locate cryptic domestic and natural container habitats for oviposition. Rearing and maintenance of Toxorhynchites under laboratory conditions are possible. These aforementioned unique characteristics led many scientists to suggest that Toxorhynchites has potential to be utilized as an alternative mosquito control method against container-inhabiting mosquito vectors of pathogens. For instance, Toxorhynchites rutilus larvae were used for the biological control of container mosquito pests in New Orleans (Focks et al. 1983), until New Orleans Mosquito Control settled on deployment of a tropical predator species (Toxorhynchites amboinensis), which yielded better coverage of both natural and artificial containers (Focks and Sackett 1985). Although the use of Toxorhynchites alone to eradicate mosquito vector populations has limited success, incorporating Toxorhynchites with other integrated mosquito control tools (e.g., insecticidal agents) may enhance the outcomes of control against the populations of mosquito vectors (Collins and Blackwell 2000, Alomar et al. 2020, Alomar and Alto 2021, 2022).
Selected References
Alomar AA, Eastmond BH, and Alto BW. 2020. The effects of exposure to pyriproxyfen and predation on Zika virus infection and transmission in Aedes aegypti. PLoS Neglected Tropical Diseases, 14: e0008846. https://doi.org/10.1371/journal.pntd.0008846
Alomar AA, and Alto BW. 2021. Mosquito responses to lethal and nonlethal effects of predation and an insect growth regulator. Ecosphere, 12: e03452. https://doi.org/10.1002/ecs2.3452
Alomar AA, and Alto BW. 2022. Evaluation of pyriproxyfen effects on Aedes aegypti and predatory mosquito Toxorhynchites rutilus (Diptera: Culicidae). Journal of Medical Entomology, 59: 585-590. https://doi.org/10.1093/jme/tjab193
Burkett-Cadena ND. 2013. Mosquitoes of the Southeastern United States. University of Alabama Press, Tuscaloosa, Alabama. 176p.
Bradshaw WE, and Holzapfel CM. 1975. Biology of tree-hole mosquitoes: photoperiodic control of development in northern Toxorhynchites rutilus (Coq.). Canadian Journal of Zoology, 53: 889-893. http://dx.doi.org/10.1139/z75-102
Bradshaw WE, and Holzapfel CM. 1983. Predator-mediated, non-equilibrium coexistence of tree-hole mosquitoes in southeastern North America. Oecologia, 57: 239-256. http://dx.doi.org/10.1007/BF00379586
Bradshaw WE, and Holzapfel CM. 1988. Drought and the organization of tree-hole communities. Oecologia, 74: 507-514. http://dx.doi.org/10.1007/BF00380047
Campos RE, and Lounibos LP. 2000a. Natural prey and digestion times of Toxorhynchites rutilus (Diptera: Culicidae) in southern Florida. Annals of the Entomological Society of America, 93: 1280-1287. http://dx.doi.org/10.1603/0013-8746(2000)093%5B1280:NPADTO%5D2.0.CO;2
Campos RE, and Lounibos LP. 2000b. Life tables of Toxorhynchites rutilus in nature in southern Florida. Journal of Medical Entomology, 37: 385-392. https://doi.org/10.1093/jmedent/37.3.385
Collins LE, and Blackwell A. 2000. The biology of Toxorhynchites mosquitoes and their potential as biocontrolagents. Biocontrol News and Information, 21: 105N-116N.
Focks DA, Hall DW, and Seawright JA. 1977. Laboratory colonization and biological observations of Toxorhynchites rutilus rutilus. Mosquito News, 37: 751-55.
Focks DA, and Boston MD. 1979. A quantified mass-rearing technique for Toxorhynchites rutilus rutilus (Coquillett). Mosquito News, 39:61 6-19.
Focks DA, Sackett SR, Dame DA , and Bailey DL. 1983. Toxorhynchites rutilus rutilus (Diptera: Culicidae). Field studies on dispersal and oviposition in the context of the biocontrol of urban container breeding mosquitoes. Journal of Medical Entomology, 20: 383-390. http://dx.doi.org/10.1093/jmedent/20.4.383
Focks DA, and Sackett SR. 1985. Some factors affecting interactions of Toxorhynchites amboinensis with Aedes and Culex in an urban environment. Pp. 55-64 in LP Lounibos, JR Rey and JH Frank (eds.) Ecology of Mosquitoes. Proceedings of a Workshop. Florida Entomology Laboratory, University of Florida, Vero Beach.
Focks DA. 2007. Toxorhynchites as biocontrol agents. Journal of the American Mosquito Control Association, 23(sp2): 118-127. http://dx.doi.org/10.2987/8756-971X(2007)23%5B118:TABA%5D2.0.CO;2
Lounibos LP. 1979. Temporal and spatial distribution, growth and predatory behaviour of Toxorhynchites brevipalpis (Diptera: Culicidae) on the Kenya coast. The Journal of Animal Ecology, 213-236. https://doi.org/10.2307/4110
Linley JR. 1987. Aerial oviposition flight of Toxorhynchites amboinensis (Diptera: Culicidae). Journal of Medical Entomology, 20: 383-390. http://dx.doi.org/10.1093/jmedent/24.6.637
Linley JR. 1989. Scanning electron microscopy of the eggs of Toxorhynchites rutilus rutilus and Tx. amboinensis. Journal of Medical Entomology, 26:1-9. https://doi.org/10.1093/jmedent/26.1.1
Lounibos LP, Escher RL, Nishimura N, and Juliano SA.1997. Long-term dynamics of a predator used for biological control and decoupling from mosquito prey in a subtropical treehole ecosystem. Oecologia 111: 189-200. http://dx.doi.org/10.1007/s004420050225
Lounibos LP, Martin EA, Duzak D, and Escher RL.1998. Daylength and temperature control of predation, body size, and rate of increase in Toxorhynchites rutilus (Diptera: Culicidae). Annals of the Entomological Society of America, 91: 308-314. http://dx.doi.org/10.1007/s004420050225
Sato S. 1961. Structure and development of the compound eye of Megarhinus towadensis Matsumura (morphological studies on the compound eye of the mosquito, no. VIII). Science Reports Tohoku University, Ser. IV. 27: 7-18.
Steffan WA, and Evenhuis NL. 1981. Biology of Toxorhynchites. Annual review of entomology, 26: 159-181. https://doi.org/10.1146/annurev.en.26.010181.001111
Schreiber ET. 2007. Toxorhynchites. Journal of the American Mosquito Control Association, 23(sp2): 129-132. https://doi.org/10.2987/8756-971X(2007)23[129:T]2.0.CO;2
Trimble RM and Smith SM. 1979. Geographic variation in the effects of temperature and photoperiod on dormancy induction, development time, and predation in the tree-hole mosquito Toxorhynchites rutilus septentrionalis (Diptera: Culicidae). Canadian Journal of Zoology, 57: 1612-1618. https://doi.org/10.1139/z79-211
Wilkerson RC, Linton YM, and Strickman D. 2021. Mosquitoes of the World (Vol. 1). Johns Hopkins University Press, 290-291pp.