The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
(Figure 1) is a neotropical mosquito that was first described in Trinidad by Dyar and Knab in 1906 (Akaratovic and Kiser 2017, ITIS 2020). Coronator means ‘crown’ in Latin, which refers to the unique crown of spines found on the tip of the larval siphon of this mosquito species. Culex coronator is a common species collected throughout Mexico, Central America, and South America (Martinez-Palacios 1952, Carpenter and Lacasse 1955). Recently expanding its range northward into the continental United States, Culex coronator has also been documented sporadically in most southern states, with widespread distributions in eastern Texas, Florida, Georgia, and South Carolina. Culex coronator’s recent rapid range expansion throughout the southern United States may pose a public health risk since St. Louis Encephalitis virus (SLEV), West Nile (WNV), and Zika virus (ZIKV) have been isolated from wild Culex coronator mosquitoes (Aitken et al. 1964; Mackay et al. 2008; Unlu et al. 2010; Elizondo-Quironga et al. 2018). Additionally, the potential for Culex coronator to transmit SLEV and WNV has been shown (Hammon and Reeves 1943; Alto et al. 2014). However, Culex coronator’s role in their transmission cycles in nature is not clearly understood.

Credit: Nathan D. Burkett-Cadena, UF/IFAS
Culex coronator is one of five species in the Culex coronator complex of the subgenus Culex (Linnaeus) 1758. The other four species in this complex are Culex camposi (Dyar) 1925, Culex ousqua Dyar 1918, Culex usquatissmus Dyar 1922 and Culex usquatus Dyar 1918. This complex is well-distributed throughout Central and South America (Akaratovic and Kiser 2017).
Distribution
Culex coronator is found throughout much of the southern United States and all countries from the U. S. to Argentina (Martinez-Palacios 1952; Carpenter and Lacasse 1955; Rossi and Vezzani 2011, Sames et al. 2021; Figure 2). Culex coronator was first collected in the U.S. in 1920 in San Benito, Texas and is currently found in Alabama, Arizona, Arkansas, Florida, Georgia, Louisiana, Mississippi, New Mexico, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, and Virginia (Dyar 1921; Darsie and Ward 2005; Smith et al. 2006; Akaratovic and Kiser 2017; Trimm et al. 2017; Sames et al. 2019; Wilke et al. 2020; Sames et al. 2021). Culex coronator was first reported in Florida in the western panhandle in 2005 and has since been documented in all 67 counties (Smith et al. 2006; Connelly et al. 2016; Sames et al. 2021; Boehmler et al. 2022).
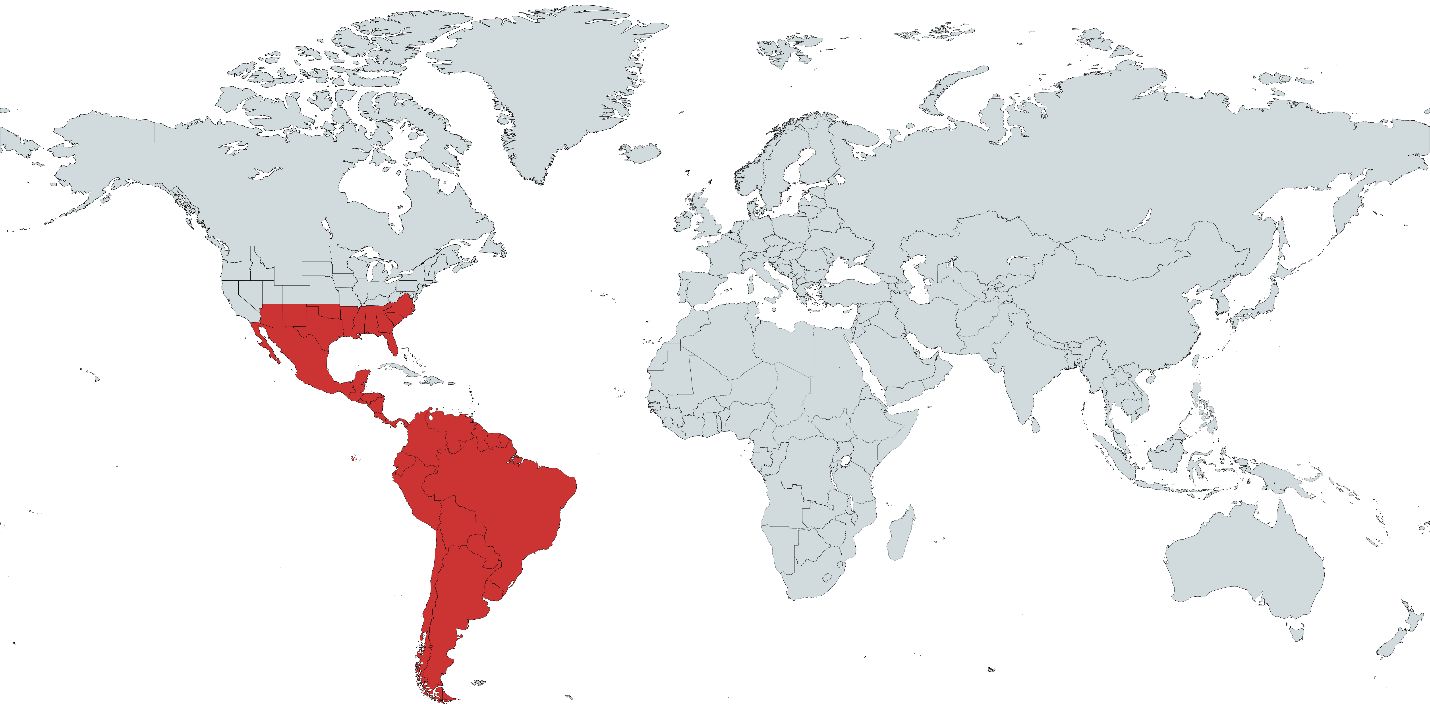
Credit: Map created using mapchart.net by Sierra M. Schluep, UF/IFAS
Description
Adults
Similar to other Culex mosquitoes, Culex coronator is brownish in color, has a blunt abdomen, and narrow wing scales. This species is a part of the Culex coronator complex, which shares morphological traits in most life stages. Molecular methods in conjunction with morphological taxonomy are typically needed to accurately identify specimens to species level. However, Laurito et al. (2018) provides a detailed assessment for distinguishing species in the complex using male genitalia. Prior studies have used the methods described in Laurito et al. (2018) to confirm that Culex coronator is the species that has expanded its distribution into the southern United States, suggesting other members of the complex have remained south of the border (Akaratovic and Kiser 2017).
Like other members of its subgenus, adult females of Culex coronator possess acrostichal setae (a row of setae arising from the central mid-line of the thorax; Figure 3), narrow, decumbent scales on their occiput (back of the head; Figure 3), and abdominal terga (the dorsal plates of each abdominal segment) with pale bands or patches along their basal boarders (Figures 3 and 4).
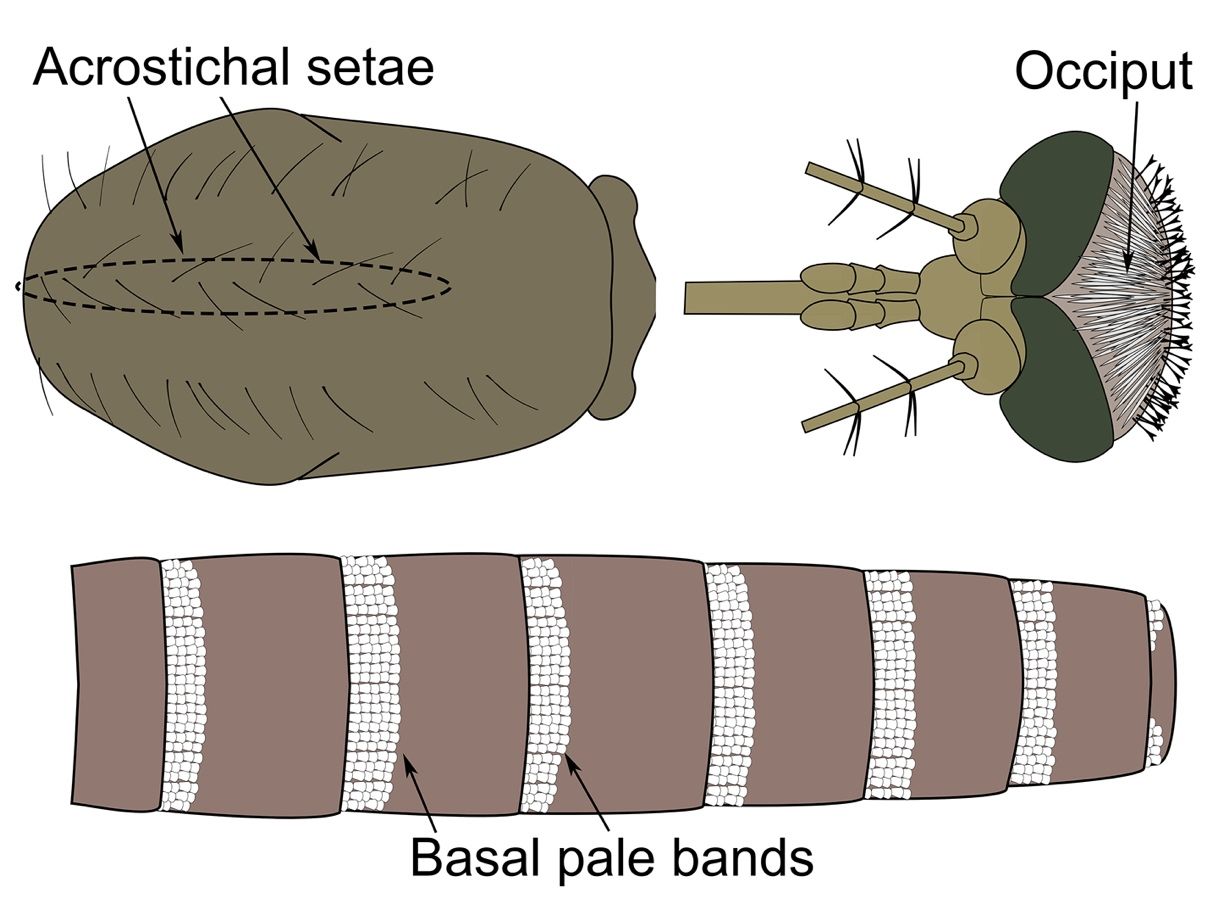
Credit: Nathan D. Burkett-Cadena, UF/IFAS

Credit: Sierra M. Schluep, UF/IFAS
Adult females of Culex coronator can be differentiated from almost all other members of the subgenus Culex in Florida by the presence of distinct basal and apical bands of pale scales on the tarsomeres of the hind legs (Figure 5). Additionally, the most apical hindleg tarsomere, hind tarsomere 5, has rings of pale scales both basally and apically with a band of dark scales in between (Figure 5). Culex coronator adults could be potentially confused with the adults of two other Culex species found in Florida that possess pale banding on the hind tarsi, Culex bahamensis Dyar and Knab and Culex tarsalis Coquillett. However, Culex coronator can be distinguished from Culex bahamensis and Culex tarsalis by the lack of a complete band of pale scales on the proboscis. Rather than a complete band, Culex coronator possesses a patch of pale scales on the underside of the proboscis (Figure 6). Also, the pleuron (lateral aspect of the thorax) of Culex coronator has alternating bands of dark and pale integument, giving the thorax a somewhat striped appearance (Figures 4 and 6).
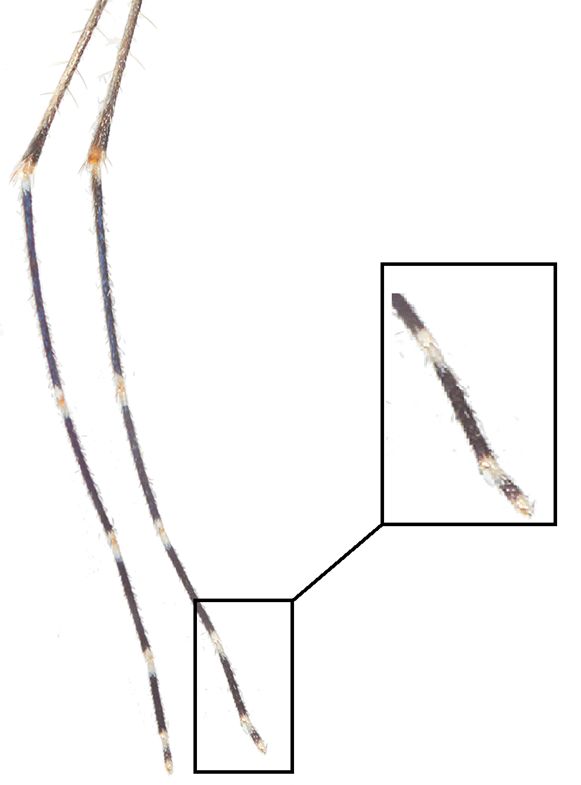
Credit: Nathan D. Burkett-Cadena, UF/IFAS

Credit: Nathan D. Burkett-Cadena, UF/IFAS
Larvae
Culex coronator larvae have an elongate siphon (siphon index: 4.5-5.5) with an apical (at the tip) ring or crown of spines (Figure 7), a feature which differentiates the larvae of this mosquito from all other mosquitoes in Florida. On the head of Culex coronator larvae, seta 6-C (a hair-like structure located on the larval head) has 3 or more branches, like other members of the subgenus Culex. The pecten, a row of small spine-like structures on the siphon, are confined to the basal thirdof the siphon (Figure 7).

Credit: Nathan D. Burkett-Cadena, UF/IFAS
Life Cycle and Biology
Culex coronator mosquitoes possess a holometabolous (complete) life cycle containing four stages: eggs, larvae, pupae, and adults.
Eggs
An adult female Culex coronator lays eggs in a single clump, forming a floating egg raft with specialized structures that allow the eggs to remain vertically upright. Eggs in a raft typically hatch shortly after embryonic development is complete because they are generally not desiccation-resistant like some mosquito species in other genera, such as Aedes or Psorophora. The source of the blood meal may influence the number of eggs in a raft laid by Culex coronator, with avian blood meals producing a greater number of eggs than mammalian blood meals (Alto et al. 2014).
Larvae
Mosquito larvae pass through four instars (stages), growing in size during each instar. The list of potential Culex coronator larval habitat sites is diverse. Larvae may be found in stagnant or slow-moving ground pools and seeps, artificial containers, ditches, culverts, tire ruts, ground depressions, and even dredge sites (Dyar and Knab 1906; Pecor et al. 2002; Varnado et al. 2012; Alto et al. 2014; Yee and Skiff 2014; Sames et al. 2019). According to Connelly et al. (2016), Culex coronator larvae are found more commonlyin open, sunlit aquatic habitatscompared to those in shaded locations. Sames et al. (2019) found that in June to early September larvae favor partially shaded areas, while in late September to the end of the larval season, larvae can be found in larval habitats in full sun.
Because of its ability to occupy artificial containers in sunlit or partially shaded areas, Culex coronator has been documented co-occurring in larval habitats with medically significant mosquito species like Aedes albopictus, Culex nigripalpus, Culex quinquefasciatus, Culex restuans, and Culex salinarius (Moulis et al. 2008; Varnado et al. 2012; Yee et al. 2012; Alto et al. 2014). Yee and Skiff (2014) found that Culex coronator larvae have higher survivorship rates and faster developmental rates when supplied with a mixture of animal waste and leaf litter as opposed to leaf litter alone. Yee and Skiff (2014) also performed a competitive study between Culex coronator, Culex quinquefasciatus, and Aedes albopictus larvae and showed that Culex coronator developed and survived at similar rates as Culex quinquefasciatus. However, Culex coronator larval survivorship was reduced by 50% when reared with Aedes albopictus larvae. These results suggest that as larvae, Culex coronator may not be likely to outcompete Aedes albopictus but should not be negatively impacted by Culex quinquefasciatus (Yee and Skiff 2014).
Pupae
The mosquito pupa is a transitional stage between the larvalstageand the adult stage. Pupaedo not eat andgenerally reside at the surface of the water except when diving to avoid potential predators.
Adults
Adult Culex coronator mosquitoes are primarily active after dusk and are not typically known to enter houses (Moulis et al. 2008; Wilke et al. 2020). Adults are primarily mammalian blood feeders. Blood meals are frequently obtained from white-tailed deer, though other mammalian hosts like racoons, humans, horses, dogs, and cats have also been identified (Roberts and Hsi 1979; Brauch 2008; Mackay et al. 2010). In addition to taking blood meals from mammals, Culex coronator occasionally feeds on birds (Mackay et al. 2010). During times of drought when suitable oviposition (egg-laying) sites may be limited, gravid Culex coronator females can delay oviposition for several weeks, typically laying eggs following a rainfall event (Mackay et al. 2010). Culex coronator mosquitoes are known to overwinter as adults (Varnado et al. 2012).
Medical Importance
Culex coronator mosquitoes have been collected from nature infected with SLEV, WNV, and ZIKV (Aitken et al. 1964; Mackay et al. 2008; Unlu et al. 2010; Elizondo-Quironga et al. 2018). Culex coronator has also been found to be a competent laboratory vector of SLEV and WNV (Hammon and Reeves 1943; Mackay et al. 2010; Alto et al. 2014). Though SLEV-, WNV-, and ZIKV-infected Culex coronator mosquitoes have been collected from the wild and their potential to transmit SLEV and WNV within the laboratory has been shown (Hammon and Reeves 1943; Aitken et al. 1964; Mackay et al. 2008; Unlu et al. 2010; Alto et al. 2014; Elizondo-Quironga et al. 2018), host feeding patterns determine the role of mosquitoes in a virus transmission cycle. So far Culex coronator has been found to predominantly take blood meals from white-tailed deer but is known to opportunistically take blood meals from both birds and humans (Roberts and Hsi 1979; Brauch 2008; Mackay et al. 2010). The opportunistic feeding on birds and humans could allow the transmission of SLEV and WNV from birds to humans and, in rare cases, of ZIKV from humans to humans. However, to better understand the role of Culex coronator in the transmission cycle of these viruses, more studies of its feeding behavior and infection prevalence in nature are needed.
Surveillance
Culex coronator larvae have been successfully collected using cups, dippers, basters, and siphons from natural and artificial ground pool habitats like flooded depressions in fields, natural and concrete roadside ditches, culverts, and abandoned swimming pools as well as artificial container habitats like water troughs, tires, plastic tubs, and barrels (Connelly et al. 2016; Sames et al. 2019 and 2021). Commonly used methods to sample Culex coronator adults include the Centers for Disease Control and Prevention (CDC) miniature light trap baited with dry ice (Figure 8), the CDC gravid trap, and the BG-Sentinel trap (Debboun et al. 2005; Varnado et al. 2005; Goddard et al. 2006; Kelly et al. 2008; Moulis et al. 2008; Noden et al. 2015; Wilke et al. 2019; Wilke et al. 2021). Adults have been captured in many areas including near woodlands, swamps, pastures, neighborhoods, and recently in deforested and highly urbanized areas (Debboun et al. 2005; Varnado et al. 2005; Smith et al. 2006; Moulis et al. 2008; Wilke et al. 2021). In the U.S., Culex coronator can be collected year-round with peak abundance usually observedin the summer and fall months (Akaratovic and Kiser 2017; Wilke et al. 2019; Sames et al. 2021).

Credit: Eva A. Buckner, UF/IFAS
Selected References
Aitken THG, Downs WG, Spence L, Jonkers AH. 1964. St. Louis encephalitis virus isolations in Trinidad, West Indies, 1953-1962. American Journal of Tropical Medicine and Hygiene 13: 450-451. https://doi.org/10.4269/ajtmh.1964.13.450
Akaratovic KI, Kiser JP. 2017. First record of Culex coronator in Virginia, with notes on Its rapid dispersal, trapping methods, and biology. Journal of the American Mosquito Control Association 33: 225-228. https://doi.org/10.2987/17-6668R.1
Alto BW, Connelly CR, O'Meara GF, Hickman D, Karr N. 2014. Reproductive biology and susceptibility of Florida Culex coronator to infection with West Nile virus. Vector-Borne and Zoonotic Diseases 14: 606-614. https://doi.org/10.1089/vbz.2013.1501
Brauch JE. 2008. The integration of mosquito avian host preference with West Nile Virus activity in wild bird and mosquito populations in Baton Rouge, Louisiana [M.S. thesis]. Louisiana State University, Baton Rouge, LA. Available from: LSU Digital Commons (etd-11112008-170918).
Boehmler MB. 2022. Culex coronator: A new species record for Monroe County, Florida. Journal of the American Mosquito Control Association 38: 96-98.
Carpenter SJ, Lacasse WJ. 1955. Mosquitoes of North America north of Mexico. University of California Press, Berkeley, pp. 360. https://doi.org/10.1525/9780520325098
Connelly C, Alto B, O'Meara G. 2016. The spread of Culex coronator (Diptera: Culicidae) throughout Florida. Journal of Vector Ecology 41: 195-199. https://doi.org/10.1111/jvec.12213
Darsie Jr RF, Ward RA. 2005. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. Gainesville, FL: University Press of Florida.
Debboun M, Kuhr DD, Rueda LM, Pecor JE. 2005. First record of Culex (Culex) coronator in Louisiana, USA. Journal of the American Mosquito Control Association 21: 455-457. https://doi.org/10.2987/8756-971X(2006)21[455:FROCCC]2.0.CO;2
Dyar HG. 1921. Ring-legged Culex in Texas (Diptera, Culicidae). Insecutor Inscitiae Menstruus 9: 32-34.
Dyar HG, Knab F. 1906. The larvae of Culicidae classified as independent organisms. Journal of the New York Entomological Society 14: 169-230, 242.
Elizondo-Quiroga D, Medina-Sánchez A, Sánchez-González JM, Eckert KA, Villalobos-Sánchez E, Navarro-Zúñiga AR, Sánchez-Tejeda G, Correa-Morales F, González-Acosta C, Arias CF, López S, Del ángel RM, Pando-Robles V, Elizondo-Quiroga AE. 2018. Zika virus in salivary glands of five different species of wild-caught mosquitoes from Mexico. Scientific Reports 16: 809. https://doi.org/10.1101/151951
Goddard J, Varnado WC, Harrison BA. 2006. Notes on the ecology of Culex coronator Dyar and Knab, in Mississippi. Journal of the American Mosquito Control Association 22: 622-625. https://doi.org/10.2987/8756-971X(2006)22[622:NOTEOC]2.0.CO;2
Hammon MD, Reeves WC. 1943. Laboratory transmission of St. Louis encephalitis virus by three genera of mosquitoes. Journal of Experimental Medicine 78: 241-253. https://doi.org/10.1084/jem.78.4.241
ITIS [Integrated Taxonomic Information System]. 2020 http://www.itis.gov. [Accessed 5 April 2020]
Kelly R, Mead D, Harrison B. 2008. Discovery of Culex coronator Dyar and Knab (Diptera:Culicidae) in Georgia. Proceedings of the Entomological Society of Washington 110: 258-260. https://doi.org/10.4289/0013-8797-110.1.258
Laurito M, Briscoe A, Almiron W, Harbach R. 2018. Systematics of the Culex coronator complex (Diptera: Culicidae): morphological and molecular assessment. Zoological Journal of the Linnean Society 182: 735-757. https://doi.org/10.1093/zoolinnean/zlx053
Mackay AJ, Kramer WL, Meece JK, Brumfield RT, Foil LD. 2010. Host feeding patterns of Culex mosquitoes (Diptera: Culicidae) in East Baton Rouge Parish, Louisiana. Journal of Medical Entomology 47: 238-248. https://doi.org/10.1603/ME09168
Mackay AJ, Roy A, Yates MM, Foil LD. 2008. West Nile Virus detection in mosquitoes in East Baton Rouge Parish, Louisiana, from November 2002 to October 2004. Journal of the American Mosquito Control Association 24: 28-35. https://doi.org/10.2987/5681.1
Martinez-Palacios A. 1952. Nota sobre la distribucion de los mosquitos Culex en Mexico (Diptera: Culicidae). Revista de la Sociedad Mexicana de Historia Natural 13: 75-87.
Moulis RA, Russell JD, Lewandowski HB, Thompson PS, Heusel JL. 2008. Culex coronator in coastal Georgia and South Carolina. Journal of the American Mosquito Control Association 24: 588-590. https://doi.org/10.2987/5766.1
Noden BH, Coburn L, Wright R, Bradely K. 2015. An updated checklist of the mosquitoes of Oklahoma including new state records and West Nile virus vectors, 2003-06. Journal of the American Mosquito Control Association 31: 336-345. https://doi.org/10.2987/moco-31-04-336-345.1
Pecor JE, Harbach RE, Peyton EL, Roberts DR, Rejmonkova E, Manguin S, Palanko J. 2002. Mosquito studies in Belize, Central America: records, taxonomic notes, and a checklist of species. Journal of the American Mosquito Control Association 18: 241-276.
Roberts DR, Hsi BP. 1979. An index of species abundance for use with mosquito surveillance data. Environmental Entomology 8: 1007-1013. https://doi.org/10.1093/ee/8.6.1007
Rossi GC, Vezzani D. 2011. An update of mosquitoes of Argentine Patagonia with new distribution records. Journal of the American Mosquito Control Association 27: 93-98. https://doi.org/10.2987/10-6082.1
Sames W, Dacko N, Bolling B, Bosworth A, Swiger S, Duhrkopf R, Burton R. 2019. Distribution of Culex coronator in Texas. Journal of the American Mosquito Control Association 35: 55-64. https://doi.org/10.2987/18-6806.1
Sames WJ, Mann JG, Kelly R, Evans CL, Varnado WC, Bosworth AB, Noden BH, Ramberg FB, Riles MT, Killingsworth D, Doyle MS. Distribution of Culex coronator in the USA. 2021. Journal of the American Mosquito Control Association 37: 1-9. https://doi.org/10.2987/21-6995.1
Smith JP, Walsh JD, Cope EH, Tennant RA, Kozak JA, Darsie RF. 2006. Culex coronator Dyar and Knab: a new Florida species record. Journal of the American Mosquito Control Association 22: 330-332. https://doi.org/10.2987/8756-971X(2006)22[330:CCDAKA]2.0.CO;2
Trimm A, Insch A, Carlson T. 2017. First record of Culex coronator in Shelby County, Tennessee. Journal of the American Mosquito Control Association 33: 345-347. https://doi.org/10.2987/17-6702.1
Unlu I, Kramer WL, Roy AF, Foil LD. 2010. Detection of West Nile virus RNA in mosquitoes and identification of mosquito blood meals collected at alligator farms in Louisiana. Journal of Medical Entomology 47: 625-633. https://doi.org/10.1093/jmedent/47.4.625
Varnado WC, Goddard J, Harrison BA. 2005. New state record of Culex coronator Dyar and Knab (Diptera: Culicidae) from Mississippi. Proceedings of the Entomological Society of Washington 107: 476-477.
Varnado W, Goddard J, Harrison B. 2012. Identification Guide to Adult Mosquitoes in Mississippi. Extension Service of Mississippi State University.
Viveiros-Rosa SG, Regis EG, Santos WC. Vector competence of Culex mosquitoes (Diptera: Culicidae) in Zika virus transmission: an integrative review. 2020. Revista Panamericana de Salud Pública 44: e7. https://doi.org/10.26633/RPSP.2020.7
Wilke A, Benelli G, Beier JC. 2020. Beyond frontiers: on invasive alien mosquito species in America and Europe. PLoS Neglected Tropical Diseases 14: e0007864. https://doi.org/10.1371/journal.pntd.0007864
Wilke A, Vasquez C, Medina J, Carvajal A, Petrie W, Beier J. 2019. Community composition and year-round abundance of vector species of mosquitoes make Miami-Dade County, Florida a receptive gateway for arbovirus entry to the United States. Scientific Reports 9: 8732. https://doi.org/10.1038/s41598-019-45337-2
Wilke ABB, Vasquez C, Cardenas G, Carvajal A, Medina J, Petrie WD, Beier JC. 2021 Invasion, establishment, and spread of invasive mosquitoes from the Culex coronator complex in urban areas of Miami-Dade County, Florida. Scientific Reports 11: 14620. https://doi.org/10.1038/s41598-021-94202-8
Yee DA, Allgood D, Kneitel JM, Kuehn KA. 2012. Constitutive differences between natural and artificial container mosquito habitats: microorganisms, resources, and habitat parameters. Journal of Medical Entomology 49: 482-491. https://doi.org/10.1603/ME11227
Yee D, Skiff J. 2014. Interspecific competition of a new invasive mosquito, Culex coronator, and two container mosquitoes, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae), across different detritus environments. Journal of Medical Entomology 51: 89-96. https://doi.org/10.1603/ME13182