The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Culex territans (Walker 1856), also known as the northern frog biting mosquito, is a mosquito species in the subfamily Culicinae, tribe Culicini, and subgenus Neoculex (ITIS 2022) (Figure 1). Located in both the New and Old Worlds, the species is found in North America, Europe, and portions of northern Africa and western Asia (Wilkerson et al. 2021). Culex territans inhabits a broad range of habitats (Knight and Stone 1977) and prefers to feed on cold-blooded animals (amphibians and reptiles), taking most of its bloodmeals from anurans (frogs and toads) (Bartlett-Healy et al. 2008a, 2009). With this unique blood feeding behavior, this species has been implicated as a vector of various microorganisms, including parasitic Trypanosoma and Hepatozoon species that infect amphibians (Bartlett-Healy et al. 2008a; 2009). Although Culex territans does not frequently bite humans, the species has been hypothesized to influence arbovirus transmission cycles. In the United States of America (USA), collected females have been found to be infected with arboviruses, including eastern equine encephalitis virus (EEEV) (Burkett-Cadena et al. 2008) and West Nile virus (WNV) (CDC 2016) acting as a potential enzootic or bridge vector for these pathogens.

Credit: Nathan Burkett-Cadena, UF/IFAS
Synonymy
Culex territans (Walker 1856) Culex saxatilis (Grossbeck 1905) Culex frickii (Ludlow 1906)
Distribution
Culex territans is a mosquito species that is located throughout much of the Northern hemisphere (Knight and Stone 1977) (Figure 2). In the New World, it occurs in Canada, USA, and Mexico. In the USA, its distribution spans from the Pacific coast to the Atlantic coast, and the species is found as far north as Alaska and as far south as Florida, but likely does not occur in southwestern states west of Texas (Darsie and Ward 2008). In the Old World, Culex territans has been collected in much of Europe, including Scandinavia, and the Mediterranean region (Wilkerson et al. 2021). Culex territans has also been reported from northern Africa and the Arabian Peninsula (Weitzel et al. 2009; Lofti 1973).
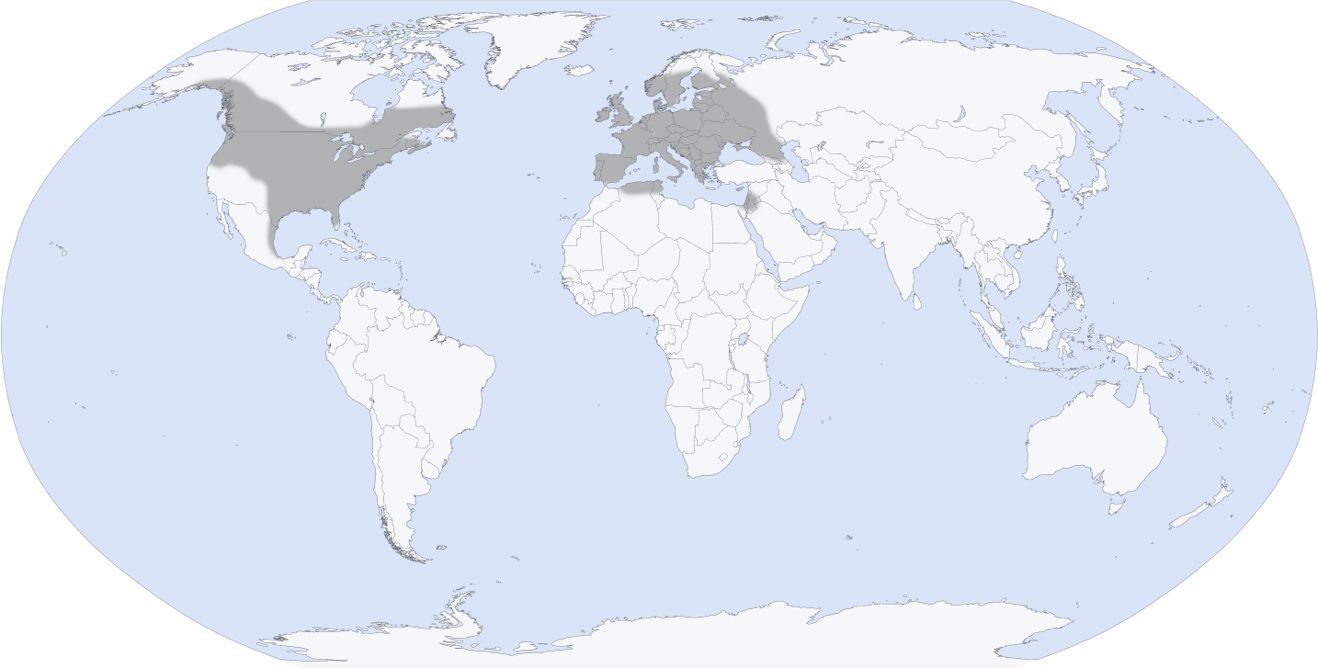
Credit: Nathan Burkett-Cadena, UF/IFAS
Description
Eggs
Like other Culex species, Culex territans lays eggs in rafts (Figure 3). Culex territans egg rafts may contain approximately 30-150 slightly curved eggs in eight parallel rows (Knab 1904; Banach 1907). The eggs of Culex territans appear smooth to the unaided eye, but under high resolution appear fine and granulate in texture (Knab 1904). Like other mosquito eggs, the eggs of Culex territans are white in color when laid, darkening to grayish brown after a few hours (Knab 1904).

Credit: Nathan Burkett-Cadena, UF/IFAS
Larvae
Larvae of Culex territans are distinguishable by their small size, prominent antennae, and rather long and thin (length about 6-7 times its width) siphon (breathing tube) (Figure 4). Larvae of Culex territans have a head that is wider than it is long and have antennae that are white with black tips (Figure 4). They can be differentiated from other Culex in Florida by the long and unbranched head setae (seta 5-C) combined with the lack of sub-dorsal setae on the siphon (Figure 4).

Credit: Nathan Burkett-Cadena, UF/IFAS
Pupae
Pupae of Culex territans are similar to that of many mosquito species. They are dark brown in color and have three main morphological characteristics of the eye, breathing trumpet, and posterior paddles used for movement (Figure 5). Pupae of Culex territans co-occur with larvae but tend to be less active and rest at the water surface unless disturbed.
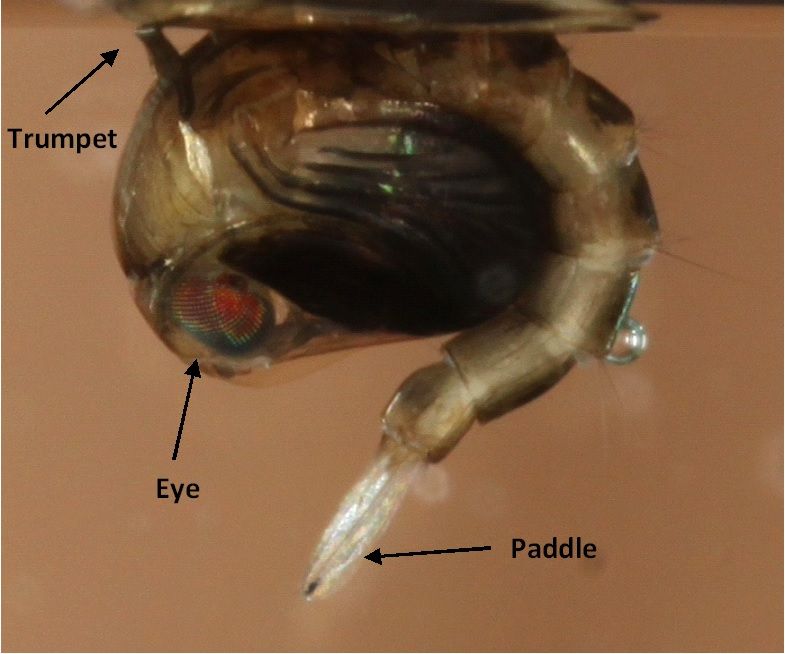
Credit: Nathan Burkett-Cadena, UF/IFAS
Adults
Adult Culex territans are a small to medium sized mosquito ranging from light to dark brown in color (Figure 6). Like other mosquito species, males contain bushy or plumose antennae while females have lightly feathered antennae. An adult female Culex territans is distinguishable from other Culex species using certain characteristics of the head, legs, and abdomen. Culex territans is the only Culex species in the eastern USA with narrow pale bands that occur apically on abdominal segments (Figure 6), whereas all other Culex species in the eastern USA have basal bands or patches of pale scales. Both the palps and proboscis of Culex territans are dark in color, with the proboscis being much longer than palps (Figure 6). The tarsi of Culex territans are dark in color, without bands of pale scales (Figure 6). The wing scales of Culex territans are dark and narrow.
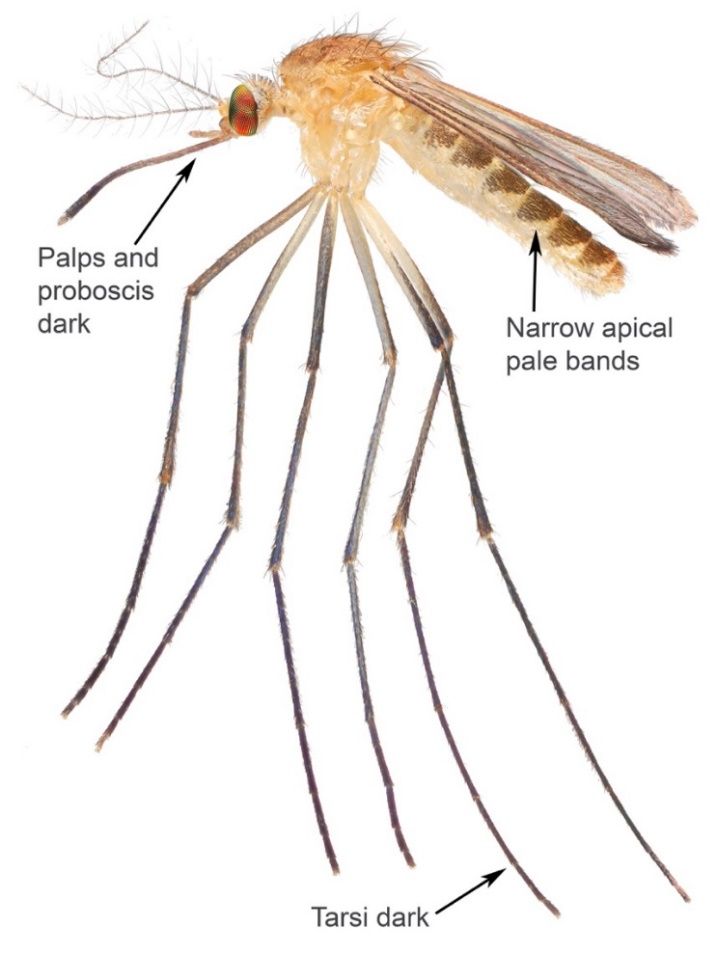
Credit: Nathan Burkett-Cadena, UF/IFAS
Life Cycle
Culex territans follows a holometabolous or full metamorphosis life cycle, going through four distinct life stages: egg, larva, pupa, and adult (Figure 7) (CDC 2020; Benach 1970). The general life cycle of Culex territans closely resembles that of other mosquitoes in the genus Culex. Females require a bloodmeal to develop eggs, which are laid in floating clusters, called egg rafts. These rafts can be often laid on aquatic vegetation or directly onto the surface of the water. Larvae hatch directly into the water and filter feed on microorganisms and detritus suspended in the aqueous environment (Merritt et al. 1992). They develop over a period of one to two weeks and then transform into pupae, which do not feed. After a few days, adults will emerge from their pupal casing and begin to search for food and mates. From field studies in New Jersey, it has been shown that mated females will feed on carbohydrate sources such as fructose and sugars (Crans 2022). Adult Culex territans do not usually fly long distances and stay close to the habitat of their preferential hosts, like amphibians, for suitable reproduction and bloodmeals (Crans 2022). From lab reared colonies, it was observed that Culex territans will feed about the same in dark and light and that adult mating in the lab environment is stenogamous, meaning that reproduction between male and female can take place without flight, where adults will mate in cages or small spaces (Benach 1970). In the winter, adult females go through a period of dormancy called diapause, to then emerge in early spring for their first bloodmeals and subsequent egg deposits (Crans 2022). The timing of diapause in Culex territans varies geographically and in some locations, like Florida and Alabama, adult Culex territans may stay active year-round continuing to blood feed during winter months (Burkett-Cadena et al. 2008; Bingham et al. 2014). Interestingly, egg hatching of Culex territans may be delayed due to the unique oviposition behavior of females, which sometimes lay eggs on emergent vegetation near the top of the water line or on a bank, relying on rainfall or rising water levels to wash the eggs onto the water surface for hatching (McIver 1969; Crans 2022). Larvae of Culex territans may also have temporal differences. In New Jersey for example, larvae of Culex territans are present throughout spring and summer and can be collected through the month of September (Crans 2022). From observations of laboratory reared Culex territans, room temperature water was ideal for larval growth and may be applicable to nature (Benach 1970).
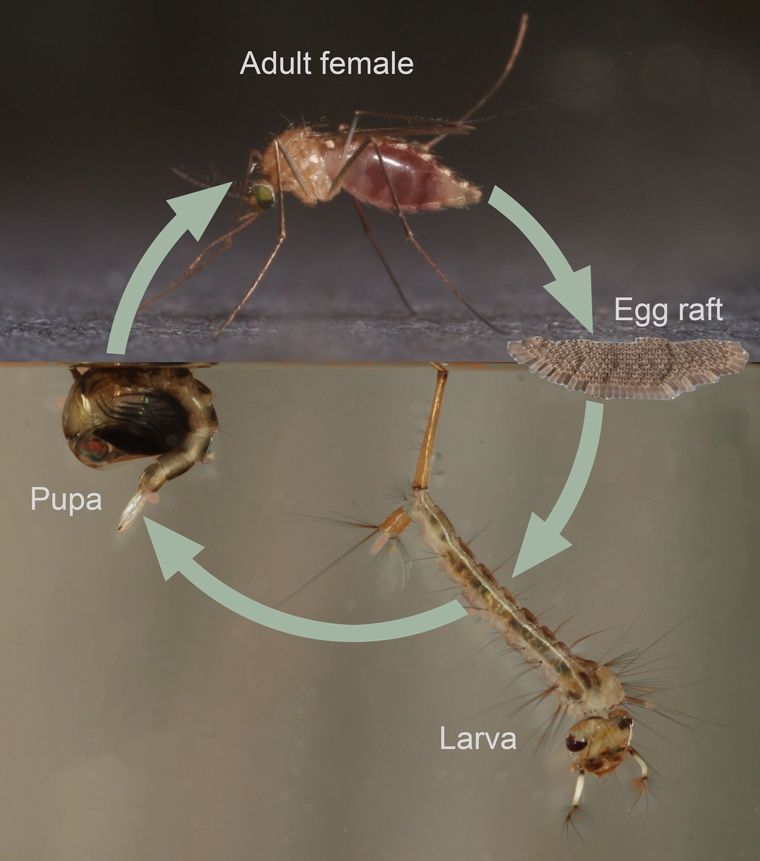
Credit: Nathan Burkett-Cadena, UF/IFAS
Habitat and Hosts
Culex territans is found in a wide variety of habitats but is most commonly associated with freshwater marshes, hardwood swamps, bogs, and ponds or streams with emergent vegetation (Joy and Clay 2002; Bartlett-Healy 2008a; Bingham et al. 2014; Burkett-Cadena et al. 2008; Botello et al. 2013; Crans 2020). All four life stages occur in such locations throughout its range, although immature stages of Culex territans have also been found in discarded tires or other artificial containers in Alabama (Qualls and Mullen 2006) and West Virginia (Joy and Clay 2002, Joy and Sullivan 2005). In Florida, Culex territans is typically found in freshwater wetlands including hardwood/cypress swamps, hardwood hammocks, pine flatwoods, and floodplain forests (Edman 1974; Bingham et al. 2014). Due to their presence in a wide variety of habitat types, Culex territans shares habitat with many other mosquito species, including those within the genera Aedes, Anopheles, Culex, and Uranotaenia, which have all been collected in similar locations (Edman 1974; Burkett-Cadena et al. 2008; Bingham et al. 2014). Like many other mosquito species, females of Culex territans also need a bloodmeal from vertebrate animals to obtain nutrients for their eggs. Culex territans across the USA and in other parts of the world have been shown to take bloodmeals from reptiles, birds, mammals (including humans), and amphibians (Table 1). Although Culex territans does feed on a variety of vertebrate hosts, it takes a large fraction (over 75%) of bloodmeals from cold blooded hosts (amphibians and reptiles), particularly anurans - frogs and toads (Figure 8; Table 1) (Edman 1974; Bartlett-Healy et al. 2009; Burkett-Cadena et al. 2008; Molaei et al. 2008; Bingham et al. 2014; Hesson et al. 2016). Due to this feeding behavior, Culex territans may be commonly encountered in water bodies with cool water temperatures where amphibians are more abundant (Crans 2022). As amphibians are an indicator species of water quality and prefer non polluted habitats, Culex territans may also likely be found in these types of areas as well (Bartlett-Healy et al. 2008a; Crans 2022). It has also been noted that due to unique temporal patterns, female Culex territans may seek reproductively active frogs, locating the calling males during specific times of their mating behavior (Bartlett-Healy et al. 2008b, Burkett-Cadena et al. 2008).
Table 1. Bloodmeal hosts (primary and secondary) of Culex territans (Walker 1856) by vertebrate class distinguished by USA state or country of collection. *References marked with asterisk break down percentages only by vertebrate class, while other references differentiate all or majority of classes by species level.

Credit: Ben Matthews, University of British Columbia, taken at Postdoctoral lab in The Rockefeller University
Medical and Veterinary Importance
Females of Culex territans have been shown to be infected with assorted parasites and pathogens that may be of medical and veterinary importance (Bartlett-Healy et al. 2008a; Bartlett-Healy et al. 2009). Due to its preference for biting amphibians, Culex territans may harbor various microorganisms that infect amphibian species, such as the filarial worm Foleyella flexicauda (Benach and Crans 1973), frog erythrocytic virus (FEV) (Gruia-Gray and Desser 1992), and various species of unicellular parasites in the phylum Apicomplexa, like Hepatazoon catesbianae (Desser et al. 1995) and Hepatazoon clamatae (Kim et al. 1998; Bartlett-Healy et al. 2008a) (Figure 9). Culex territans may also have a role in the transmission of various Trypanosoma species; such as Trypanosoma ranarum (Barta and Desser 1984; Bartlett-Healy et al. 2009) and Trypanosoma rotatorium (Desser et al. 1973; Bartlett-Healy et al. 2009) among amphibian hosts, or like Trypanosoma culicavium (Votýpka et al. 2012) among avian hosts (Figure 9). In the United States, Culex territans females have also been found to be infected with two medically important arboviruses, EEEV (Burkett-Cadena et al. 2008) and WNV (CDC 2016), which circulate among birds in enzootic cycles. Although Culex territans predominantly bites amphibians, studies of mosquito feeding behavior have reported Culex territansspecimenswith human and avian bloodmeals(Table 1). It is therefore possible that this species plays a minor role in transmitting arboviruses among wild vertebrate hostsas well as to humans, makingthismosquito a potential enzootic vectorand bridge vector of EEEV and WNV (White et al. 2011; Bingham et al. 2014; Burkett-Cadena et al. 2014; Shepard et al. 2016).
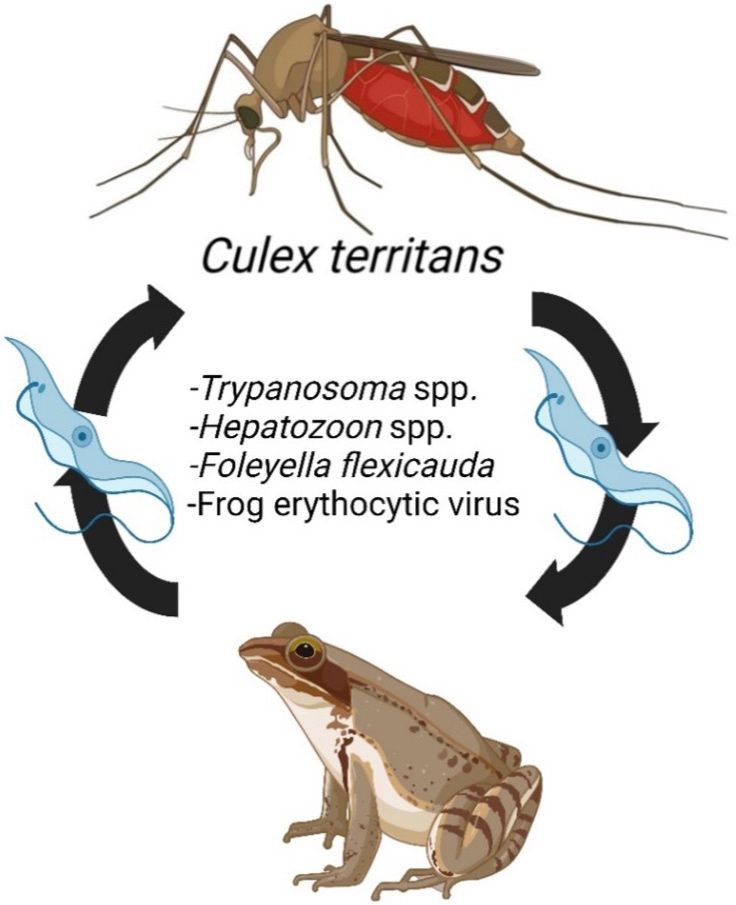
Credit: Figure created in BioRender.com (Toronto, ON, CA) by Chanakya Bhosale, UF/IFAS
Surveillance and Management
Because Culex territans mainly feeds on amphibians, the surveillance and management of the mosquito species is not a priority of mosquito control or public health agencies. However, as Culex territans has been found infected with viruses like WNV and EEEV and has been shown to occasionally feed on humans, it is crucial to understand more about this species’ biology to improve surveillance practices for public/veterinary health and research purposes. Larvae of Culex territans are commonly found and collected using a standard mosquito dipper in their typical habitats by skimming and dipping into emergent vegetation in the water or in small cavities (Bartlett-Healy et al. 2008a; Crans 2022). As larvae prefer to rest in shaded water, looking for shady areas on sunny days may improve efforts to collect Culex territans larvae (Crans 2022). From studies utilizing adult mosquito collections, Culex territans has been sampled with high efficiency using resting-site aspiration, as well as with carbon dioxide-baited CDC miniature light traps or vegetation sweeps to a lesser degree (Savage et al. 2007; Burkett-Cadena et al. 2008; Molaei et al. 2008; Bingham et al. 2014). In these studies, resting adult Culex territans have been collected from microhabitats such as tree cavities, storm water sewers, understory vegetation, and researcher-made resting shelters (Savage et al. 2007; Burkett-Cadena et al. 2008; Molaei et al. 2008; Bingham et al. 2014). Female Culex territans have been found to be attracted to amphibian hosts via phonotaxis, using vocalizations of frog species to locate these animals for bloodmeals (Bartlett-Healy et al., 2008b). Although more research is needed to fully understand host attraction for this species, phonotaxis could be exploited as a method of attracting Culex territans for surveillance or management purposes.
Selected References
Barta JR, Desser SS. 1984. Blood parasites of amphibians from Algonquin Park, Ontario. Journal of Wildlife Diseases 20: 180-189. https://doi.org/10.7589/0090-3558-20.3.180
Bartlett-Healy K, Crans W, Gaugler R. 2008a. Temporal and spatial synchrony of Culex territans (Diptera: Culicidae) with their amphibian hosts. Journal of Medical Entomology. 45: 1031-1038. https://doi.org/10.1093/jmedent/45.6.1031
Bartlett-Healy K, Crans W, Gaugler R. 2008b. Phonotaxis to amphibian vocalizations in Culex territans (Diptera: Culicidae). Annals of the Entomological Society of America 101: 95-103. https://doi.org/10.1603/0013-8746(2008)101[95:PTAVIC]2.0.CO;2
Bartlett-Healy K, Crans W, Gaugler R. 2009. Vertebrate hosts and phylogenetic relationships of amphibian trypanosomes from a potential invertebrate vector, Culex territans Walker (Diptera: Culicidae). Journal of Parasitology 95: 381-387. https://doi.org/10.1645/GE-1793.1
Benach JL. 1970. Observations of a colony of Culex territans Walker. Proceedings of the New Jersey Mosquito Extermination Association 57: 70-75.
Benach JL, Crans WJ. 1973. Larval development and transmission of Foleyella flexicauda Schacher and Crans, 1973 (Nematoda: Filarioidea) in Culex territans. Journal of Parasitology 59: 797-800. https://doi.org/10.2307/3278408
Bingham AM, Burkett-Cadena ND, Hassan HK, McClure CJW, Unnasch TR. 2014. Field investigations of winter transmission of eastern equine encephalitis virus in Florida. American Journal of Tropical Medicine and Hygiene 91: 685-693. https://doi.org/10.4269/ajtmh.14-0081
Botello G, Golladay S, Covich A, Blackmore M. 2013. Immature mosquitoes in agricultural wetlands of the coastal plain of Georgia, USA: effects of landscape and environmental habitat characteristics. Ecological Indicators 34: 304-312. https://doi.org/10.1016/j.ecolind.2013.05.018
Burkett-Cadena ND, Graham SP, Hassan HK, Guyer C, Eubanks MD, Katholi CR, and Unnasch TR. 2008. Blood feeding patterns of potential arbovirus vectors of the genus Culex targeting ectothermic hosts. American Journal of Tropical Medicine and Hygiene 79: 809-815. https://doi.org/10.4269/ajtmh.2008.79.809
Burkett-Cadena ND, Bingham AM, Porterfield C, and Unnasch TR. 2014. Innate preference or opportunism: mosquitoes feeding on birds of prey at the Southeastern Raptor Center. Journal of Vector Ecology 39: 21-31. https://doi.org/10.1111/j.1948-7134.2014.12066.x
CDC - Centers for Disease Control and Prevention. 2016. Mosquito species in which West Nile virus has been detected, United States, 1999-2016. https://www.cdc.gov/westnile/resources/pdfs/MosquitoSpecies1999-2016.pdf
CDC - Centers for Disease Control and Prevention. 2020. Life Cycle of Culex Species Mosquitoes. Centers for Disease Control and Prevention. https://www.cdc.gov/mosquitoes/about/life-cycles/culex.html
Crans WJ. 2022. Culex territans Walker. https://vectorbio.rutgers.edu/outreach/species/terr.htm
Darsie, R.F., Ward, R.A., 2008. Correction to: identification and geographical distribution of the mosquitoes of North America, North of Mexico. J. Am. Mosq. Control Assoc. https://doi.org/10.2987/5706.1
Desser SS, Hong H, Martin DS. 1995. The life history, ultrastructure, and experimental transmission of Hepatozoon catesbianae n. comb., an apicomplexan parasite of the bullfrog, Rana catesbeiana and the mosquito, Culex territans in Algonquin Park, Ontario. Journal of Parasitology 212-222. https://doi.org/10.2307/3283922
Desser SS, McIver SB, Ryckman A. 1973. Culex territans as a potential vector of Trypanosoma rotatorium. I. Development of the flagellate in the mosquito. Journal of Parasitology 59: 353-358. https://doi.org/10.2307/3278833
Edman JD. 1974. Host-feeding patterns of Florida mosquitoes. 3. Culex (Culex) and Culex (Neoculex). Journal of Medical Entomology 11: 95-104. https://doi.org/10.1093/jmedent/11.1.95
Grossbeck JA. 1905. New species of culicidae. The Canadian Entomologist 37: 359-360. https://doi.org/10.4039/Ent37359-10
Gruia-Gray J, Desser SS. 1992. Cytopathological observations and epizootiology of frog erythrocytic virus in bullfrogs (Rana catesbeiana). Journal of Wildlife Diseases 28: 34-41. https://doi.org/10.7589/0090-3558-28.1.34
Hesson JC, Schäfer M, Lundström JO. 2016. First report on human-biting Culex pipiens in Sweden. Parasites and Vectors 9: 632. https://doi.org/10.1186/s13071-016-1925-3
Heym EC, Kampen H, Schäfer M, Walther D. 2019. Mosquito bloodmeal preferences in two zoological gardens in Germany. Medical and Veterinary Entomology 33: 203-212. https://doi.org/10.1111/mve.12350
Irby WS, Apperson CS. 1988. Hosts of mosquitoes in the coastal plain of North Carolina. Journal of Medical Entomology 25: 85-93. https://doi.org/10.1093/jmedent/25.2.85
ITIS - Integrated Taxonomic Information System. 2022. ITIS - Report: Culex territans. https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=126501#null
Joy JE, Clay JT. 2002. Habitat use by larval mosquitoes in West Virginia. The American Midland Naturalist 148: 363-375. https://doi.org/10.1674/0003-0031(2002)148[0363:HUBLMI]2.0.CO;2
Joy JE, Sullivan SN. 2005. Occurrence of tire inhabiting mosquito larvae in different geographic regions of West Virginia. Journal of the American Mosquito Control Association 21(4): 380-386. https://doi.org/10.2987/8756-971X(2006)21[380:OOTIML]2.0.CO;2
Kim B, Smith TG, Desser SS. 1998. The life history and host specificity of Hepatozoon clamatae (Apicomplexa: Adeleorina) and ITS-1 nucleotide sequence variation of Hepatozoon species of frogs and mosquitoes from Ontario. Journal of Parasitology 84: 789-797. https://doi.org/10.2307/3284589
Knight K, Stone A. 1977. A catalog of the mosquitoes of the world. The Thomas Say Foundation 6: 611.
Knab F. 1904. The Eggs of Culex territans Walker. Journal of the New York Entomological Society 12: 246-248.
Lotfi MD. 1973. Iranian species of Genus Culex (Culicinae: Díptera). ÏI. Report of four species of larvae (including three new records) and 14 adult species. Bulletin de la Société de Pathologie Exotique 66: 204-207.
Ludlow CS. 1906. MOSQUITO NOTES.-No. 4. The Canadian Entomologist 38: 132-134. https://doi.org/10.4039/Ent38132-4
Merritt RW, Dadd RH, Walker ED. 1992. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annual Review of Entomology. 37: 349-374. https://doi.org/10.1146/annurev.en.37.010192.002025
McIver SB. 1969. Notes on the biology of Culex territans Walker. Mosquito News 29: 135 -136. https://doi.org/10.1111/j.1752-7325.1969.tb02823.x
Molaei G, Andreadis TG, Armstrong PM, Diuk-Wasser M. 2008. Host-feeding patterns of potential mosquito vectors in Connecticut, USA: molecular analysis of bloodmeals from 23 species of Aedes, Anopheles, Culex, Coquillettidia, Psorophora, and Uranotaenia. Journal of Medical Entomology 45: 1143-1151. https://doi.org/10.1093/jmedent/45.6.1143
Qualls WA, Mullen GR. 2006. Larval survey of tire-breeding mosquitoes in Alabama. Journal of the American Mosquito Control Association. 22(4): 601-608. https://doi.org/10.2987/8756-971X(2006)22[601:LSOTMI]2.0.CO;2
Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, Anderson M, Charnetzky D, McMillen L, Unnasch EA, Unnasch TR. 2007. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002-2003. Vector Borne and Zoonotic Diseases 7: 365-386. https://doi.org/10.1089/vbz.2006.0602
Shepard JJ, Andreadis TG, Thomas MC, Molaei G. 2016. Host associations of mosquitoes at eastern equine encephalitis virus foci in Connecticut, USA. Parasites and Vectors 9: 474. https://doi.org/10.1186/s13071-016-1765-1
Walker F. 1856. Insecta Britannica: Diptera. Reeve and Benham.
Weitzel T, Collado A, Jöst A, Pietsch K, Storch V, Becker N. 2009. Genetic differentiation of populations within the Culex pipiens complex and phylogeny of related species. Journal of the American Mosquito Control Association 25: 6-17. https://doi.org/10.2987/08-5699.1
White G, Ottendorfer C, Graham S, Unnasch TR. 2011. Competency of reptiles and amphibians for eastern equine encephalitis virus. American Journal of Tropical Medicine and Hygiene 85: 421-425. https://doi.org/10.4269/ajtmh.2011.11-0006
Wilkerson RC, Linton Y-M, Strickman D. 2021. Mosquitoes of the World. Johns Hopkins University Press.
Votýpka J, Szabová J, Rádrová J, Zídková L, Svobodová M. 2012. Trypanosoma culicavium sp. nov., an avian trypanosome transmitted by Culex mosquitoes. International Journal of Systematic and Evolutionary Microbiology 62: 745-754. https://doi.org/10.1099/ijs.0.032110-0