The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
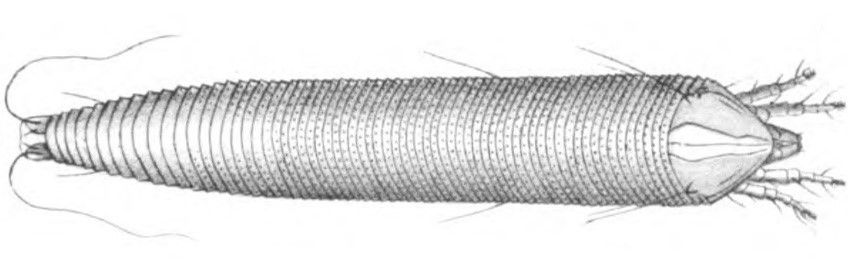
Credit: Nalepa (1906)
Aceria hibisci, the hibiscus erineum mite, is a mite in the family Eriophyidae and can be a pest on ornamental Hibiscus rosa-sinensis plants. Eriophyid mites are small worm-like mites that feed on plants by sucking out the cellular contents from leaves and stems. During feeding, they release chemicals that cause the formation of abnormal plant growths like galls. Galls are tumor-like growths that occur on plants and can be induced by viruses, fungi, or arthropods such as gall wasps or mites.
Synonymy and Taxonomy
Aceria hibisci was collected initially from galled Hibiscus rosa-sinensis plants in Fiji and described as Eriophyes hibisci Nalepa, 1906. In 1966, Keifer reclassified the mite under the genus Aceria and distinguished it from a similar species based on the lack of a curved carina (a ridge present in the exoskeleton) at the rear of the shield (the large portion on top of the “head” of the mite) (Keifer, 1966).
Distribution and Habitat
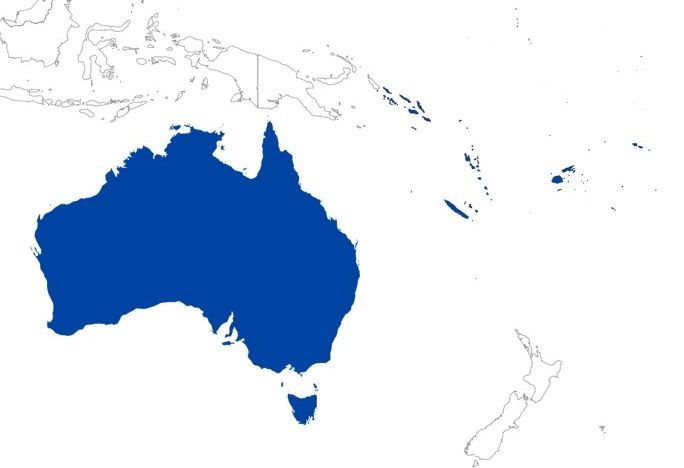
Credit: Mikinley Weaver, University of Florida (As of July 01, 2023)
Aceria hibisci is not currently present in the mainland United States and is primarily known from the Pacific islands, including Fiji (Nalepa, 1906), Hawaii (Hara et al., 2001), American Samoa (Mua and Joshi, 2014), and seven other Western Pacific countries (Keifer, 1946; Williams and Watson, 1990; Welbourn et al., 2009). In the Caribbean, the mite has been documented in five territories/countries, including Cuba (De la Torre and Martinez, 2004), Puerto Rico, and Jamaica (Welbourn et al., 2009).
Credit: Mikinley Weaver, University of Florida
These mites are very small, making it difficult to travel between separate host plants independently. However, their size allows them to be distributed on the wind or as hitchhikers on animals or other debris as it moves between host plants. They most likely act as phoretic mites, meaning they primarily depend on other insects/animals to transfer between host plants (Jeppson et al., 1975; Hara et al., 2001; Brown et al., 2021).

Credit: Mikinley Weaver, University of Florida
Description & Life Cycle
The life cycle of Aceria hibisci has not been directly studied. While no experimental studies involving this species were found while searching the literature, it shares many biological similarities to other eriophyid mites, and their life cycles are likely to be similar. As such, the following explanations of the life cycle are mostly generalizations and may be proven inaccurate after further observations and studies are performed.
Eggs
The translucent eggs are tiny (20–60 µm) and impossible to distinguish without a microscope (Brown et al., 2021). The eggs are deposited directly on the leaves or buds of the plant and hatch a few days after being deposited (Manson and Oldfield, 1996).
Like many mites and even some insects, female Aceria hibisci lay two types of eggs – fertilized (diploid) and unfertilized (haploid) eggs. Unfertilized eggs produce males, while fertilized eggs produce females (Oldfield and Michalska, 1996). This enables a population to theoretically be started by a single female under the right conditions (Michalska et al., 2009). Females likely lay between 1 and 5 eggs daily, with egg counts of other species totaling 50 to 80 eggs (Jeppson et al. 1975).
Nymphal Stages
Aceria hibisci has two nymphal stages (Jeppson et al., 1975). These nymphs feed on the same plants as the adults and fill the same ecological niche. Morphologically, first-stage nymphs may have a different number of microtubercules from adults, but second-stage nymphs closely resemble adults. The only distinguishing features for second-stage nymphs are that they are smaller and lack external genitalia, features common to all nymph stages.
Adults
Only 150 microns in size, the adults are generally colorless and wormlike in appearance with two pairs of legs (Nalepa, 1906) – a characteristic unique to the eriophyid mites. Their bodies are divided into three poorly divided segments: the rostrum (the snout-like front of the head), the cephalothorax (the fused head and thorax), and the abdomen. Generally, the cephalothorax is modified into a large section above the rostrum, called a shield, with some parts indistinguishably merged with the abdomen. This shield forms the primary basis for many species identifications, but so do more minor morphological features such as setae (hairs) and other small body protrusions. Because of their small size, even the largest adults are only visible using at least 10X magnification, such as a hand lens or a microscope.
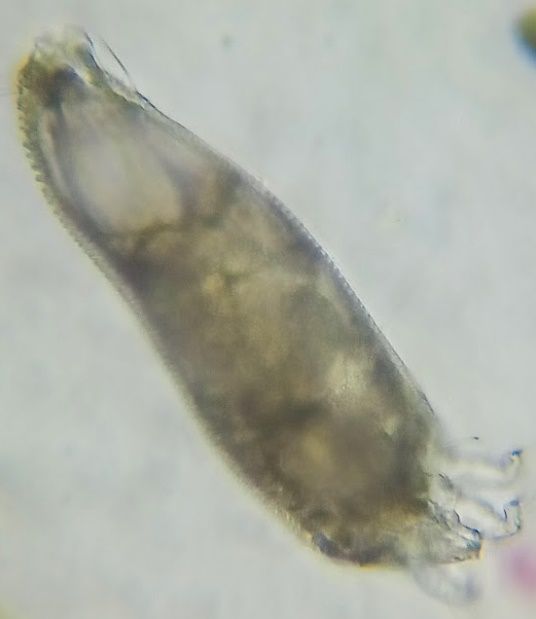
Credit: Mikinley Weaver, University of Florida
The adult stage of Aceria hibisci is the stage in which reproduction occurs. Reproduction occurs through the transference of external spermatophores (a secreted packet of sperm within a protective casing or sack). Males will deposit spermatophores in areas that females frequent. Females will then sense the spermatophore, move onto it, and deposit the sperm into their spermathecae (her sperm storage organ). Because fertilization does not occur when the two sexes are in contact, the males must place large quantities of spermatophores in hopes that the females will find them as they pass through the area (Jeppson et al., 1975). Once fertilized, adult females will lay the eggs, and the males will continue to deposit spermatophores.
In total, the entire life cycle of the mites is relatively short, with a complete cycle occurring in less than three weeks (Jeppson et al., 1975; Hara et al., 2001). This cycle - egg, nymph 1, nymph 2, and adult – is considered simple. However, many eriophyid mites have a complex life history which includes an alternation of generations, referred to as the complex lifestyle.
In the complex lifestyle, eriophyid mites will have males and two different kinds of females, protogynes and deutogynes. Protogynes, or primary females, are females who resemble males and live in times of favorable conditions. These females are the primary egg layers of the colony. Deutogynes (secondary females), in contrast, are morphologically different from the males/protogynes and have distinct coloration as well - making them difficult to identify correctly. These females appear during times of less favorable conditions, especially before winter. These females will mate but cannot lay eggs during the same year they breed. Instead, they must undergo a period of diapause and winter chilling before they lay eggs again the following spring. After laying eggs, the deutogynes will die off, leaving a colony consisting only of nymphal males and protogynes. In this way, these females act as living reserves for the species when unfavorable conditions would otherwise kill them off (Jeppson et al., 1975).
This complex cycle is mentioned because it is unknown whether it occurs in Aceria hibisci. The complex life cycle is known from eriophyid mites in temperate regions, and Aceria hibisci is currently limited to tropical regions. However, it may be that this life history trait is conserved and not seen because the necessary environmental cues are absent. Should this be the case, introductions of Aceria hibisci could prove to be much more invasive than previously considered.
Host Plants
Aceria hibisci is explicitly found on the species Hibiscus rosa-sinensis, a plant in the family Malvaceae (the Mallow family, which includes cotton and okra). Aceria hibisci has been reportedly found on the Blue Mahoe plant, Talipariti elatus (de la Torre and Martinez, 2004); Okra, Abelmoschus esculentus (Hara et al., 2001; de la Torre and Martinez, 2004; Welbourn et al., 2009); and other unspecified members of the genus Hibiscus (Hara et al., 2001; Welbourn et al., 2009). However, the reports of host species other than Hibiscus rosa-sinensis are likely attributable to erroneous identifications or a misreading of the original source materials, as in Mua and Joshi (2014).

Credit: Mikinley Weaver, University of Florida
In support of this hypothesis, experimental trials have not seen any galling of other Malvaceae plants grown near infested Hibiscus rosa-sinensis plants. Looking at a plant within the family but not the same genus, Welbourn et al. (2009) reported that Malvaviscus arobreus (Wax Mallow) have been grown near mite-infested Hibiscus plants but remained free of infection.
It is plausible that a host shift to closely related members of the genus Hibiscus could occur, such as to Hibiscus arnottianus, which is known to hybridize readily with Hibiscus rosa-sinensis, but no shift has been observed. In an evaluation of the galls on Hibiscus arnottianus plants growing within 10 meters of heavily infested Hibiscus rosa-sinensis plants, the Hibiscus arnottianus galls showed no presence of eriophyid mites indicating that Aceria hibisci was not the cause of those galls despite the proximity (personal observation, October 2022). While this does not explicitly demonstrate that host transference has not occurred anywhere, it raises the possibility that the mites are not readily host-switching.
Without a thorough review of collected specimens or further experimental evidence, the range of Aceria hibisci’s host plants remains unconfirmed. However, based on the evidence above, it is argued that the species should continue to be described as host-specific, as in Robbs and Peracchi (1972) and Navia et al. (2021).
Damage
Hibiscus erineum mites feed on Hibiscus rosa-sinensis plants and cause the formation of galls. Galls caused by these mites are unique in appearance. Starting flat, these galls eventually grow to form open galls with erinea, or hair-like growths on the galls. These erinea can create dense habitats, providing shelter for the mites and causing the galls to appear “velvety”–an expected result of mites from the family Eriophyidae (Jeppson et al., 1975).
Initially, these galls will form on the leaves, presenting as only cosmetic damage. If the infestation worsens, the mites can rapidly multiply and spread to other parts of the plant. This will lead to galls on the younger, softer portions of the stems and the flowers. Having galls on the flowers can cause the flowers to delay opening or even drop without opening, leading to a loss of aesthetic value. Once levels have reached this severity, the damage often leads to stunting of the plant and susceptibility to disease because it cannot outgrow the damage caused by the mites.
Economic Importance
Under the assumption that Aceria hibisci is host specific to Hibiscus rosa-sinensis, the economic damage is limited to ornamental plants only. The worldwide ornamental flower market is estimated to be valued at 36 billion US dollars (Gabbelini and Scaramuzzi, 2022), with Hibiscus spp. especially valued for the beauty of their flowers, their ease of propagation, and the association between the flowers and tropical areas such as Hawaii. Hibiscus rosa-sinensis is notable as it is the most common Hibiscus species sold and encountered as an ornamental. Although the mite does not decimate the crops of Hibiscus growers, any plants sold must either be treated to prevent the spread of the mites or sold with obvious mite deformities, limiting the plant’s commercial value. Additionally, landowners will often spend money on improper chemical treatments for the mites. Because of these things, the mites can represent a significant economic loss for all Hibiscus growers.
Because of the geographical spread of the mite, it is in a prime position to be introduced into Florida. Many areas where it is already present have commercial agricultural trade with Florida and can act as a source population for an accidental introduction. Because of this risk, it is important to be aware of the mite and the damage it can induce. If you notice galling on your Hibiscus plants and live in an area where the mite is not currently introduced, it is crucial that you take proper steps to identify and quarantine your plants to prevent the spread of the mite.
Management
The management of Hibiscus erineum mites is best accomplished through a combination of cultural and biological controls, with chemical controls being the least effective method (Brown et al., 2021).
Biological Control
Predatory mites–fast-moving mites visible to the naked eye–are known to enter the open-ended galls and feed on Hibiscus erineum mites (Hara et al., 2001). These populations can be augmented to improve their efficacy. Surveys have shown that mites in the family Phytoseiidae, specifically Phytoseius intermedius, are specifically associated with Aceria hibisci and may represent the optimal mites for use as biological controls (Welbourn et al., 2009). Predatory mites can be released directly onto the infected plants and will quickly find their prey.
Cultural Control
One of the simplest methods for reducing mite loads, cultural controls involve pruning severely infested portions of the plants and then disposing of them in a way that prevents the spread of the mites (i.e., burning, burying, or disposing of them in sealed plastic bags). Though these methods are temporary mite load reducers, the infestations will usually return without combining this with other techniques, such as biological control (Welbourn et al., 2009). Additionally, cuttings should not be taken from areas where infestations are already occurring, and mite-resistant hybrids should be employed in areas where the mite loads are expected to be high (Hara et al., 2009).
Mechanical Control
The best mechanical control is physically isolating infected plants and not allowing them within the same space as uninfected plants.
Chemical Control
The recommendation of miticides is difficult as they must be used according to the label, and the approved options are constantly changing while showing variable levels of efficacy (Brown et al., 2021; Dively et al., 2022). Some general insecticides can kill eriophyid mites, but the formation of galls can make applications difficult by providing areas for the mites to hide and avoid the chemicals (Van Leeuwen et al., 2009) while necessitating frequent reapplications (Brown et al., 2021). Additionally, miticide applications can harm the beneficial mite populations, which work to reduce the mite levels naturally. Because of these reasons, chemical controls are only recommended after all other means have proven ineffective.
Selected References
Brown MS, Blubaugh CK, Chong JH. 2021. Biology and management of eriophyid mites in turfgrass. Journal of Integrated Pest Management 12: 25. https://doi.org/10.1093/jipm/pmab020
Dively GP, Hartman ME, Ochoa R. 2022. Population dynamics of eriophyid mites and evaluation of different management practices on Timothy Grass. Journal of Economic Entomology 115: 602-610. https://doi.org/10.1093/jee/toac004
Gabellini S, Scaramuzzi S. 2022. Evolving consumption trends, marketing strategies, and governance settings in ornamental horticulture: A grey literature review. Horticulturae 8: 234. https://doi.org/10.3390/horticulturae8030234
Guo J-F, Li H-S, Wang B, Xue X-F, Hong X-Y. 2015. DNA barcoding reveals the protogyne and deutogyne of Tegolophus celtis sp. nov. (Acari: Eriophyidae). Experimental and Applied Acarology 67: 393-410. https://doi.org/10.1007/s10493-015-9953-9
Hara A, Tsuda D, Tavares J, Yogi J, Hensley D. 2001. Hibiscus Erineum Mite. Insect Pests.
Jeppson LR, Keifer HH, Baker EW. 1975. Mites injurious to economic plants. https://doi.org/10.1525/9780520335431
Keifer HH. 1946. Eriophyid studies XVI. Bulletin for the Department of Agriculture of California. 35: 39-44. https://doi.org/10.1093/jee/39.5.563
Keifer HH. 1966. Eriophyid studies. State Bureau of Entomology, California Department of Agriculture. 22 pp.
Lindquist EE, Sabelis MW, Bruin J (Eds.). 1996. Eriophyoid mites: their biology, natural enemies, and control. Elsevier, Amsterdam. 790 pp.
Manson DCM, Oldfield GN. 1996. Life forms, deuterogyny, diapause and seasonal development. Eriophyoid mites: their biology, natural enemies, and control. Elsevier, Amsterdam. pp. 173-183. https://doi.org/10.1016/S1572-4379(96)80009-1
Marini F, Weyl P, Vidović B, Petanović R, Littlefield J, Simoni S, de Lillo E, Cristofaro M, Smith L. 2021. Eriophyid mites in classical biological control of weeds: Progress and challenges. Insects 12: 513. https://doi.org/10.3390/insects12060513
Michalska K, Skoracka A, Navia D, Amrine JW. 2010. Behavioural studies on eriophyoid mites: an overview. Experimental and Applied Acarology 51: 31-59. https://doi.org/10.1007/978-90-481-9562-6_3
Monfreda R, Nuzzaci G, Lillo ED. 2007. Detection, extraction, and collection of eriophyoid mites. Zootaxa 1662: 35-43.
Mua M, Joshi RC. 2014. Galling of the Chinese hibiscus (Hibiscus rosa-sinensis L.) in Samoa. Fiji Agricultural Journal 54: 54-58.
Nalepa A. 1906. Über zwei neue Eriophyiden den von den fidischiinseln. Journal of Economic Biology 1: 147-151.
Navia D, Duarte ME, Flechtmann CHW. 2021. Eriophyoid mites (Acari: Prostigmata) from Brazil: an annotated checklist. Zootaxa 4997: 1-152. https://doi.org/10.11646/zootaxa.4997.1.1
Oldfield GN, Michalska K. 1996. Spermatophore deposition, mating behavior and population mating structure. Eriophyoid mites: their biology, natural enemies, and control. Elsevier, Amsterdam. pp. 185-198. https://doi.org/10.1016/S1572-4379(96)80010-8
de la Torre P, Martinez H. 2004. Lista de los ácaros Eriofioideos (Acari: Prostigmata: Eriophyoidea) de Cuba. Revista Ibérica de Aracnología 9: 123-126.
Van Leeuwen T, Witters J, Nauen R, Duso C, Tirry L. 2010. The control of eriophyoid mites: state of the art and future challenges. Experimental and Applied Acarology 51: 205-224. https://doi.org/10.1007/978-90-481-9562-6_11
Welbourn C, Rodrigues JC, Peña JE. 2009. The Hibiscus Erineum Mite, Aceria hibisci (Acari: Eriophyidae) a new introduction in the Caribbean and a potential threat to Florida's hibiscus. https://doi.org/10.32473/edis-in777-2008
Williams DJ, Watson GW. 1990. The scale insects of the tropical South Pacific region. 3: The soft scales Coccidae and other families. CAB International, Wallingford. 267 pp.