The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Synonymy
Dermestes sexdentatus Börner, 1776
Bostrichus pinastri Bechstein, 1818
Ips sexdentatus junnanicus Sokanovskii, 1959
Taxonomy
Ips sexdentatus belongs to the large genus of conifer feeding bark beetles Ips DeGeer (Insecta: Coleoptera: Curculionidae: Scolytinae: Ipini) (Wood 1982, Cognato and Sun 2007). The genus is characteristic by having the elytral declivity always steeply sloped, excavated, and armed by several spines around the margins.
Introduction
Bark beetles are among the most important forest insects. Numerous species have been introduced to non-native regions globally, with some becoming invasive and causing substantial damage. Ips sexdentatus, also known as the six-toothed spruce bark beetle, primarily colonizes Mediterranean pines. Occasionally, it also colonizes other conifer species, like spruce, but rarely. Although not yet recorded outside its native range, it has been included on the 2023 Priority Pest List designed by USDA’s Animal and Plant Health Inspection Service (APHIS) due to its high potential to invade the North American continent.
Ips sexdentatus primarily feeds on phloem (inner bark, phloeophagous) and inadvertently vectors several fungi, including Ophiostoma spp. (Ascomycota). One such fungus is Ophiostoma brunneo-ciliatum, which is known for causing “blue-stain” discoloration in wood and timber (Villari et al. 2012, Linnakoski et al. 2012). Similar to other Ips species, Ips sexdentatus males initially colonize trees, constructing chambers in the phloem to attract females for mating. Typically, multiple females, usually two to five, mate with a single male. Mated females excavate lengthwise galleries starting from the mating chamber to lay eggs.

Credit: Yiyi Dong, UF/IFAS
Identification
The body length ranges from 5.0 to 8.0 mm (~1/8 – 3/8 in) (Figure 1, A-B). The protibia of all Ips (tibia of the first leg) bears four or five separated denticles on the outer margin (without counting the apical spine, the “uncus”) (Douglas et al. 2019). Ips sexdentatus is characterized by following unique combination of morphological features: In females, the heads are characterized by transverse carina (elevated ridge); the elytral declivity (the sloping part of the elytra) is gradual, and each lateral margin displays six denticles, with the fourth spine being the largest (Douglas et al. 2019).
Credit: Yiyi Dong, UF/IFAS
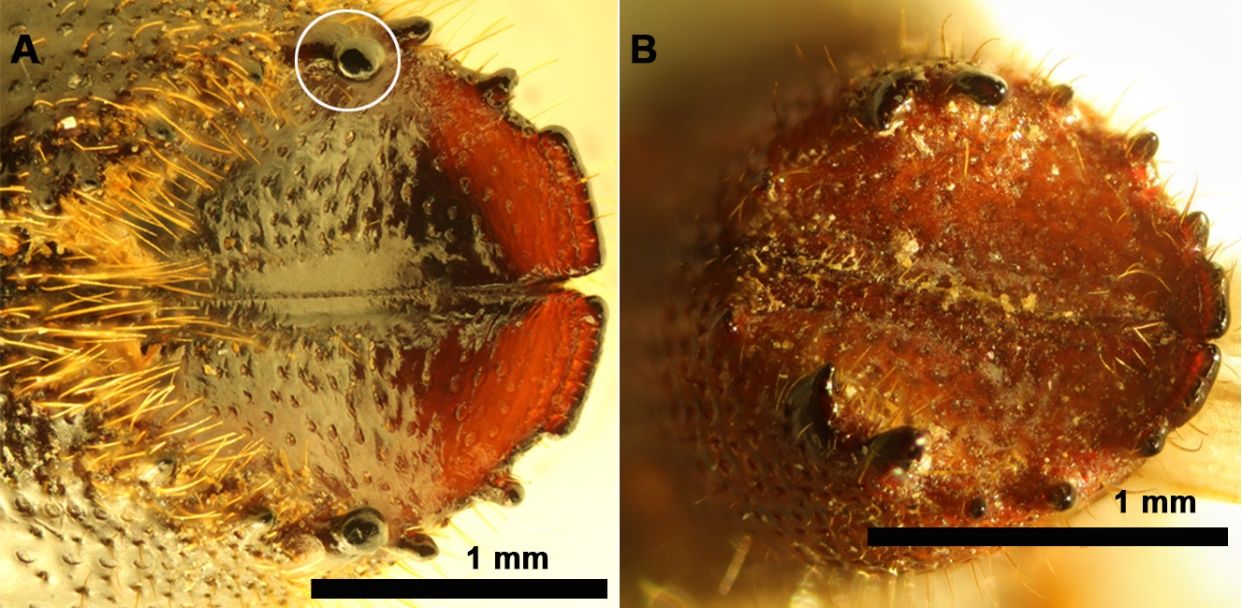
This species may be confused with several Ips species native to North America, such as Ips calligraphus Germar and Ips apache Lanier (Insecta: Coleoptera: Curculionidae: Scolytinae). While all three species feature six spines on each side of the elytral declivity, several morphological characteristics help distinguish Ips sexdentatus. Ips sexdentatus is larger than most other species in this genus (Sellamuthu et al. 2021). There is a size overlap between smaller Ips sexdentatus specimens and larger individuals of Ips calligraphus and Ips apache so, additional features must be considered. One such feature is the presence of the largest spine at the fourth position (from eltral summit to elytral apex) on the elytral declivity (Figure 2, A-B) (Douglas et al. 2019), although this feature is variable in Ips calligraphus. Moreover, Ips sexdentatus is missing punctures on interstriae 2 and 3 on the basal third of the elytral top; the punctures are present in Ips calligraphus and Ips apache (Douglas et al. 2019).
Ips sexdentatus galleries are very similar to those of most other Ips and cannot be easily used as a diagnostic character. The system consists of three to six slightly sinuous maternal tunnels, which start from the large mating chamber. The sinuous larval galleries with a roundish chamber at the end are usually shorter than the maternal galleries (Faccoli 2015).
Distribution
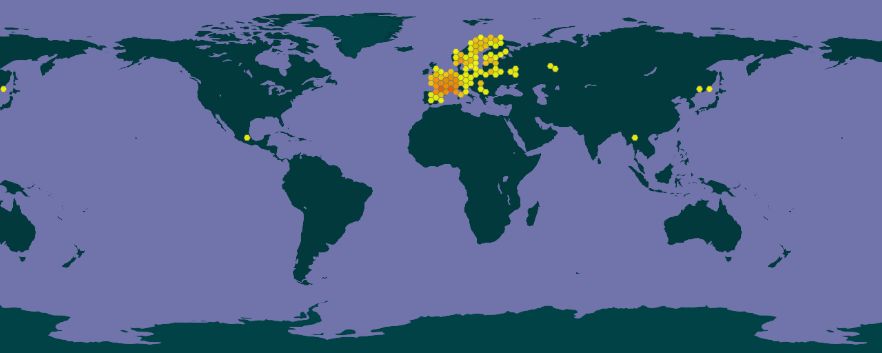
Ips sexdentatus naturally occurs in a wide range of ecosystems across Eurasia and has been documented in 49 countries (Douglas et al. 2019).
Credit: © OpenStreetMap contributors, © OpenMapTiles, GBIF
Life Cycle
As in most beetles, the life cycle of Ips sexdentatus includes four stages (egg, larva, pupa, and adult). Females start to lay eggs one to seven days post-mating, with eggs developing into larvae after approximately three weeks. Both the larval and pupal stages each span roughly two weeks in duration (Sarikaya et al. 2012). A female Ips sexdentatus typically produces up to 60 offspring, maintaining an approximately balanced sex ratio (Pineau et al. 2017). Typically, females of Ips sexdentatus create niches along their galleries under the bark to lay single eggs in each niche. The species is known for its fast reproduction, able to produce two or more generations annually (Rossi et al. 2009, Ozcan et al. 2011, Jeger et al. 2017). For example, in Turkey, which has a similar latitude to the Eastern US, the first generation usually begins around April to May. By the end of June, newly molted adults fly away in search of new host plants, signaling the start of the second generation. A third generation often begins towards the end of September (Ozcan et al. 2011). However, this generational frequency can vary and is influenced by several biotic and abiotic factors, including temperature and the availability of host plants. For instance, populations in the tropical mountains in Thailand have more generations annually compared to those in the temperate Western Europe.
Hosts
Ips sexdentatus has been recorded from many conifer tree species within the Pinaceae family. These include various species of pine (Pinus), such as Pinus brutia Ten, Pinus sylvestris L., Pinus armandii Franch, Pinus heldreichii Christ., and Pinus nigra Arn; spruce (Picea), including Picea orientalis (L.) Link; fir (Abies), including Abies nordmanniana (Stev.) Spach; as well as some species of cedar (Cedrus) and larch (Larix) (Wood and Bright 1992, Cognato 2015).
Damage
Ips sexdentatus typically is not a primary reason for tree death, infesting declining and dead trees rather than actively killing healthy ones. Under normal circumstances, its economic impact is limited to the introduction of ophiostomatoid fungi, which can discolor wood (Jeger et al. 2017), Davydenko et al. 2021). This beetle often co-colonizes trees with other bark beetle species, such as Tomicus piniperda L. (Faccoli 2015). However, Ips sexdentatus can become a more significant threat during periods of high population density, contributing to tree dieback and economic losses. A notable example of this was observed in southwest France in 1999, where Ips sexdentatus colonized trees damaged by a windstorm (Rossi et al. 2009).
There have also been occasional reports of Ips sexdentatus infesting and purportedly killing pine or spruce trees in Turkey and Europe (Fernández 2006, Zink et al. 2019, Knížek and Véle 2022). While the species typically targets weakened trees, impacted by factors such as wildfire, windstorms, and drought (Fernández 2006, Rossi et al. 2009, Davydenko et al. 2021), there is evidence to suggest that in conditions of large population buildup, Ips sexdentatus may also attack healthy trees (Fernández 2006).
Diagnosis
Trees infected by Ips sexdentatus exhibit typical symptoms of bark beetle colonization, including branch dieback, yellowing needles, entrance holes, frass or sawdust, and resin flow on the bark. Wood discoloration is also a sign of attack by Ips sexdentatus, as these beetles often carry the blue stain fungi.
Invasion status
Ips sexdentatus is regulated as a quarantine pest in several countries, such as some European island countries (Ireland and Cyprus) (Jeger et al. 2017). It has also been recently added to the USDA's Animal and Plant Health Inspection Service's 2023 Priority Pest List, highlighting its high potential for invasion to the North American continent.
Invasion pathway
Trade or transportation of large coniferous wood sections with bark, including some wood packaging materials, represents significant risks as a dispersal pathway for Ips sexdentatus across different regions. The movement of such materials can inadvertently provide a refuge for Ips sexdentatus, facilitating their spread to new areas. The likelihood of establishment and subsequent ecological impact is considerably higher if these beetles are introduced into areas where their primary host, spruce, is prevalent.
Smaller plant parts, wood without bark, and live plants are less likely to facilitate the spread of Ips sexdentatus. Human-mediated movement, particularly through wood products and wood packaging, is identified as the primary pathway for the species’ introduction and spread beyond its native range (Jeger et al. 2017).
Management
Management of secondary conifer-infesting bark beetles, like Ips sexdentatus, primarily involves preventing tree stress. Trees in plantations are more susceptible to attacks by secondary borers during stress periods, particularly if they have not been conditioned to handle environmental stressors. Practices such as thinning and stand diversification are crucial for adapting these trees to such stressors.
For monitoring purposes, Ips sexdentatus can be attracted to traps baited with the aggregation pheromone components: ipsenol, ipsdienol, and 2-methyl-3-buten-2-ol.
In its natural range, Thanasimus formicarius Linnaeus (Insecta: Coleoptera: Cleridae) and Temnoscheila caerulea Olivier (Insecta: Coleoptera: Trogossitidae) are natural predators of the larvae and adults of Ips sexdentatus, which could potentially be used as biological controls where these insects are absent (Warzée et al. 2006, Özcan and Kocoglu 2018, Bracaliniet al. 2021). Congeners of these generalist subcortical predators are already present and common in North America.
To effectively mitigate the risk of Ips sexdentatus and other bark beetles invading from overseas, it is critical to adopt both preventative and responsive strategies. Closing the invasion pathways, as emphasized by Lovett et al. (2016), is a fundamental step. This involves regulation and inspection of international trade, especially of wood products and packaging, and proactive monitoring. As Rabaglia et al. (2018) suggest, these systems should be capable of early detection, enabling rapid response to any potential infestation. Such efforts are not only essential for managing existing beetle populations but are also the most effective measures currently available to prevent future invasions. Managing invasive bark beetles requires collaboration between regulatory agencies, scientific communities, and industry stakeholders.
References
Bracalini M, Croci F, Ciardi E, Mannucci G, Papucci E, Gestri G, Tiberi R, Panzavolta T. 2021. Ips sexdentatus mass-trapping: Mitigation of its negative effects on saproxylic beetles larger than the target. Forests, 12: 175. https://doi.org/10.3390/f12020175
Cognato AI. 2015. Biology, systematics, and evolution of Ips. In Bark beetles: biology and ecology of native and invasive species. Edited by F.E. Vega and R.W. Hofstetter. Elsevier, San Diego, California. Pp. 351-370. https://doi.org/10.1016/B978-0-12-417156-5.00009-5
Cognato AI, Sun JH. 2007. DNA based cladograms augment the discovery of a new Ips species from China (Coleoptera: Curculionidae: Scolytinae). Cladistics 23:539-551. https://doi.org/10.1111/j.1096-0031.2007.00159.x
Davydenko K, Vasaitis R, Elfstrand M, Baturkin D, Meshkova V, Menkis A. 2021. Fungal communities vectored by Ips sexdentatus in declining Pinus sylvestris in Ukraine: focus on occurrence and pathogenicity of Ophiostomatoid species. Insects 12:1119. https://doi.org/10.3390/insects12121119
Douglas HB, Cognato AI, Grebennikov V, Savard K. 2019. Dichotomous and matrix-based keys to the Ips bark beetles of the World (Coleoptera: Curculionidae: Scolytinae). Canadian Journal of Arthropod Identification. https://doi.org/10.3752/cjai.2019.38
Faccoli M. 2015. European bark and ambrosia beetles: types, characteristics and identification of mating systems. WBA Handbooks 5:160. ISSN:1973-7815
Fernández MMF. 2006. Colonization of fire-damaged trees by Ips sexdentatus (Boerner) as related to the percentage of burnt crown. Entomologica Fennica, 17(4): 381-386. https://doi.org/10.33338/ef.84361
Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Jaques Miret JA, MacLeod A. 2017. Pest categorisation of Ips sexdentatus. EFSA Journal 15:e04999. https://doi.org/10.2903/j.efsa.2017.4999
Knížek M, Liška J, Véle A. 2022. Efficacy of synthetic lures for pine bark beetle monitoring. Journal of Forest Science, 68(1): 19-25. https://doi.org/10.17221/139/2021-JFS
Linnakoski R, De Beer ZW, Niemelä P, Wingfield MJ. 2012. Associations of conifer-infesting bark beetles and fungi in Fennoscandia. Insects 3:200-227. https://doi.org/10.3390/insects3010200
Lovett GM, Weiss M, Liebhold AM, Holmes TP, Leung B, Lambert KF, Orwig DA, Campbell FT, Rosenthal J, McCullough DG, Wildova R, Ayres M P, Canham C D, Foster D R, LaDeau Shannon L, Weldy T. 2016. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecological applications, 26:1437-1455. https://doi.org/10.1890/15-1176
Ozcan G E, Eroglu M, Akinci HA. 2011. Use of pheromone-baited traps for monitoring Ips sexdentatus (Boerner)(Coleoptera: Curculionidae) in oriental spruce stands. African Journal of Biotechnology 10:16351-16360. https://doi.org/10.5897/AJB11.1709
Özcan GE, Kocoglu N. 2018. Feeding preferences of the rearing of Thanasimus formicarius (L.)(Coleoptera, Cleridae). Alinteri Journal of Agriculture Science 33:215-220. https://doi.org/10.28955/alinterizbd.449574
Rabaglia RJ, Cognato AI, Hoebeke ER, Johnson CW, LaBonte JR, Carter ME, Vlach JJ. 2019. Early detection and rapid response: a 10-year summary of the USDA Forest Service program of surveillance for non-native bark and ambrosia beetles. American Entomologist, 65:29-42. https://doi.org/10.1093/ae/tmz015
Rossi JP, Samalens JC, Guyon D, van Halder I, Jactel H, Menassieu P, Piou D. 2009. Multiscale spatial variation of the bark beetle Ips sexdentatus damage in a pine plantation forest (Landes de Gascogne, Southwestern France). Forest Ecology and Management 257:1551-1557. https://doi.org/10.1016/j.foreco.2008.12.012
Sarikaya O, Avci M, Yildirim S. 2012. Flight activity and biology of Ips sexdentatus Boerner in Black pine (Pinus nigra Arnold) forests of Isparta, Turkey. Pages 597-604 in International Scientific Conference, Forests in the future–Sustainable use, risks and challenges, Institute of Forestry, Belgrade, Republic of Serbia. ISBN: 8680439334
Sellamuthu G, Amin S, Bílý J, Synek J, Modlinger R, Sen MK, Chakraborty A, Roy A. 2021. Reference gene selection for normalizing gene expression in Ips sexdentatus (Coleoptera: Curculionidae: Scolytinae) under different experimental conditions. Frontiers in Physiology 12:752768. https://doi.org/10.3389/fphys.2021.752768
Villari C, Battisti A, Chakraborty S, Michelozzi M, Bonello P, Faccoli M. 2012. Nutritional and pathogenic fungi associated with the pine engraver beetle trigger comparable defenses in Scots pine. Tree physiology, 32:867-879. https://doi.org/10.1093/treephys/tps056
Warzée N, Gregoire JC, Jactel H, Menassieu P, Malosse C. 2006. Semiochemical diversity and niche partitioning among Scolytids and the generalist bark-beetle predator, Thanasimus formicarius (Coleoptera: Cleridae). Forest Insect Population Dynamics and Host Influences. http://hdl.handle.net/2013/ULB-DIPOT:oai:dipot.ulb.ac.be:2013/270943
Wood SL. 1982. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. https://biostor.org/reference/239409
Wood SL, Bright Jr DE. 1992. A catalog of Scolytidae and Platypodidae (Coleoptera). part 2: taxonomic index. Great Basin Naturalist Memoirs 13:1-1553. https://biostor.org/reference/143868
Zink FA, Tembrock LR, Timm AE, Gilligan TM. 2019. A duplex ddPCR assay for simultaneously detecting Ips sexdentatus and Ips typographus (Coleoptera: Curculionidae) in bulk trap samples. Canadian Journal of Forest Research 49:903-914. https://doi.org/10.1139/cjfr-2019-0047