The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The American palm cixiid, Haplaxius crudus, is a common insect species that belongs to the order Hemiptera, family Cixiidae. It is widespread and abundant in the state of Florida, but also occurs as far north as South Carolina and as far west as Texas. Furthermore, it is common throughout the Caribbean basin. This insect feeds on a wide variety of palm species as adults while the immature stages feed on a diversity of grass species (over 40). This species is of high economic concern due to its ability to transmit the lethal yellowing (LY) phytoplasma (Howard and Thomas 1980) and is also the putative vector of lethal bronzing (LB) phytoplasma, both of which are fatal diseases of palms.
Description
Adults
Adults of Haplaxius crudus (Figure 1) are the most commonly encountered life stage and are approximately 2.5 to 3 mm (0.1 in) long. Females are slightly longer and more robust than males with a sword-like ovipositor. Both males and females are a light yellow color, however females in general are darker in color with the abdomen having an olive/brownish hue. Males are a much brighter yellow over the entire body with parts of the abdomen either being orange or green. However, color is inconclusive for species determination and confirmation can only be attained by observing features present in the male genitalia (Figure 2) using a microscope.
Nymphs
There are five nymphal instars (life stage between each time the insect sheds its exoskeleton) that are generally white in color with grey hue to the head and thorax and covered in many wax-producing pits (Figure 3). First instar: First instar nymphs are approximately 0.5 mm (0.02 in) long, second instars: approximately 1 mm (0.04 in) long, third instars: approximately 1.5 mm (0.06 in), fourth instars: approximately 2 mm (0.08 in), and fifth instar nymphs: approximately 2.5 mm (0.1 in) long.
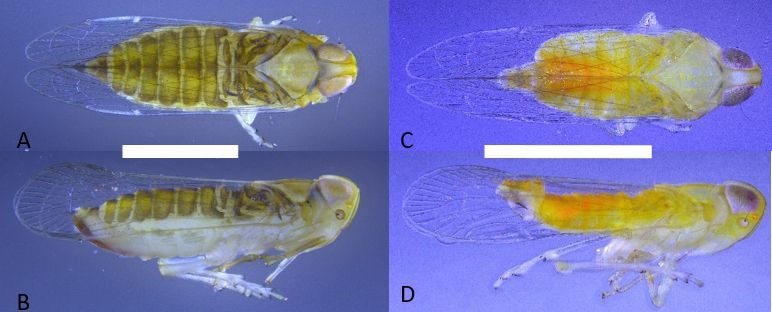
Credit: Brian Bahder
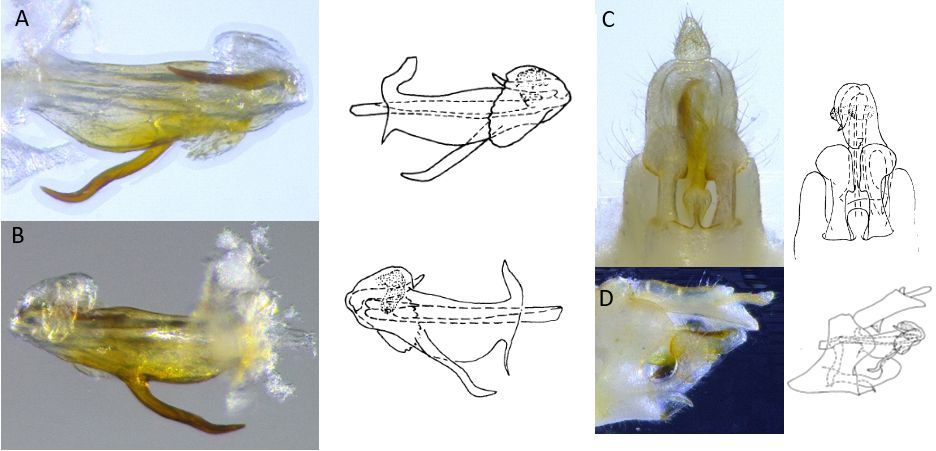
Credit: Brian Bahder
Life Cycle
The immature lifestages of Haplaxius crudus are small and rather cryptic due to their preferred habitat (thatch layer of grasses). Eggs are laid at the base of grasses, often in the thatch layer of common turfgrass species. It takes anywhere from 10 to 20 days for the eggs to hatch. The duration of each instar is approximately one week under warm conditions, going from egg to adult in 1.5 months (at 30°C (86°F)) (Tsai and Kirsch 1978). All nymphal instars are strict grass feeders. After adults emerge, they migrate into palm canopies where they feed exclusively on palm foliage for the duration of their adult life. When mating occurs, it is initiated in the palm canopy where males and females find each other using vibrational signals on the leaf surface. Mating pairs can easily be found on palm foliage during the correct time of year (usually spring, however mating can occur year-round in the southern half of the state). After copulation, females migrate into appropriate grass hosts to lay eggs (an average of 13 but ranging from 5 to 31).

Credit: Brian Bahder
Host Range
As adults, Haplaxius crudus only feeds on palm (Arecaceae) foliage (Figure 4). Within the Arecaceae, it has a wide host range, having been collected on more than 30 species of palms. While it does seem to be more readily collected on coconut palm (Cocos nucifera), this could be due to surrounding grassy habitat generally associated with coconut plantations rather than true host preference. Nymphs have been documented from over 40 different species of grasses and sedges throughout the Neotropics. However, in Florida the preferred host in the urban landscape is St. Augustinegrass (Reinert 1980).

Credit: De-fen Mou
Economic Importance
Haplaxius crudus is considered to be of major economic importance. In the 1970s and 1980s, Haplaxius crudus was confirmed as the vector of lethal yellowing (LY) (Figure 5), a disease caused by the 16SrIV-A phytoplasma that is lethal to a wide variety of economically important palm species in Florida. Throughout the Caribbean basin, LY has resulted in the death of millions of coconut palms, thus causing severe economic losses to both ornamental and agricultural sectors of many Caribbean nations. Haplaxius crudus is also the putative vector of lethal bronzing (LB) in Florida (Figure 5). It is important to note that in the absence of phytoplasma diseases, Haplaxius crudus causes no damage to palms that it feeds on. Due to the biology of the phytoplasmas, only a small proportion (less than 1%) of Haplaxius crudus carry the phytoplasma in the wild but, due to the widespread distribution and abundance of Haplaxius crudus, this is enough to cause persistent disease pressure in the urban landscape and in nurseries.

Credit: Brian Bahder
Sampling and Management
Currently, the most time- and cost-effective method to sample for adults of Haplaxius crudus is by placing yellow sticky traps in the canopy of palms that are accessible from the ground (Figure 6). During peak adult activity, this method can capture over 300 individuals after about two weeks’ time. Adults can also be readily collected by sweep-netting palm canopies if living insects are needed. Finally, adults readily come to lights at night, so light-trapping with a mercury vapor lamp is also a useful collection method. Nymphs are far more difficult to reliably sample due to grasses covering a much large ground area than palms. Because of their cryptic habitat, yellow sticky traps, sweep netting and light trapping are not effective for sampling. Currently, the optimal strategy for collecting nymphs is by use of a Berlese funnel where clumps of grass and a thin layer of dirt/roots are dug up (Figure 6) and placed upside down inside the funnel and a light is turned on to heat the samples. As the grass dries, nymphs try to escape the light/heat and move downward, falling into a container with 95% ethanol. This method is labor intensive and not cost effective and is only recommended for specific research needs, however, it may be needed by nurseries in the future to supplement management programs.
As part of the palm phytoplasma diagnostic clinic at the UF/IFAS Fort Lauderdale Research and Education Center (FLREC), processing of yellow sticky traps to determine presence/absence of Haplaxius crudus and if the local population carries the phytoplasma is now offered as an available service to all interested stakeholders. Sample submission forms and instructions can be found at www.bahderlab.com under the “Diagnostic Services” tab.
Current recommendations for management primarily involve weed/grass management in nurseries, systemic treatments of palms with dimethoate, and contact treatments against nymphs using diazinon (Howard and McCoy 1980). Both dimethoate and diazinon are commercially available. For grass management in nurseries, the current recommendation is to remove any and all grasses and replace with a non-monocot cover-crop. Research is ongoing to determine what chemical compounds and formulations can be used effectively in grasses in nurseries to control populations of Haplaxius crudus.

Credit: Brian Bahder
Selected References
Howard, F.W. and McCoy, R.E., 1980. Reduction in spread of mycoplasma-like organism-associated lethal decline of the palm, Veitchia merrilli, by use of insecticides. Journal of Economic Entomology, 73(2), pp. 268-270.
Howard, F.W. and Thomas, D.L., 1980. Transmission of palm lethal decline to Veitchia merrillii by a planthopper Myndus crudus. Journal of Economic Entomology, 73(5), pp. 715-717.
Reinert, J.A., 1980. Phenology and density of Haplaxius crudus (Homoptera: Cixiidae) on three southern turfgrasses. Environmental Entomology, 9(1), pp. 13-15.
Tsai, J.H. and Kirsch, O.H., 1978. Bionomics of Haplaxius crudus (Homoptera: Cixiidae). Environmental Entomology, 7(2), pp. 305-308.