The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The coconut mite, Aceria guerreronis Keifer, attacks young fruits of the coconut palm, Cocos nucifera L., to which it is almost exclusively confined. The mites are small, with the largest stage around 250 µm in length, but they often build up extremely large and dense populations, in which case their feeding causes scarring and distortion of the fruits and may cause premature fruit drop. It is one of the worst arthropod pests of coconut palm, whether grown as a crop tree or as an ornamental and is the only eriophyid mite that is a serious pest of coconut palm. It is distributed in many tropical countries where coconuts grow. In Florida it is very prevalent on coconut palms on the Florida Keys and occurs sporadically on the mainland.
Three additional eriophyid mites occur on coconut palms in Florida, including (Keifer), Acrinotus denmarki Keifer, and Amrinus coconuciferae (Keifer). These are found principally on the leaves, usually in scarce populations that do not cause significant damage. There is a world total of at least 12 eriophyid mite species associated with coconut palms.
The vernacular name coconut mite has also been applied to both Acathrix trymatus and Raoiella indica Hirst (Tenuipalpidae) in addition to Aceria guerreronis. The latter species, which is highly destructive to coconut palm foliage, is native to Southern Asia but was recently found in several islands of the Caribbean and thus is a threat to coconut palms in Florida and throughout the region.
Distribution
The coconut mite was described by the eminent acarologist Hartford Keifer in 1965 from specimens collected in Guerrero, Mexico. The same year it was found near Rio de Janeiro, Brazil. Subsequently, it was found in many countries of Tropical America and in West Africa. It is controversial whether it is native to the Eastern or Western Hemisphere. Botanists have accumulated evidence that the coconut palm evolved in the South Pacific Region, and during ancient times was spread by people along the coastal regions of Asia and ultimately into Africa and brought from West Africa to the American Tropics in the 1500s by Spanish and Portuguese colonists. It seems unlikely that the coconut mite could have been introduced into the New World along with the earliest introductions of the coconut itself and remained at undetectable populations for more than four centuries. However, some coconut growers and other observers in the Caribbean have asserted that damage to coconuts typical of coconut mite was occasionally observed long before the mite was authoritatively discovered in their respective countries. In fact, in 1984, when the species was positively identified for the first time in the continental United States by H. A. Denmark from specimens collected by F. W. Howard from coconuts on Sugarloaf Key, FL, coconut mite damage was common on the Florida Keys and many residents had been familiar with it for years.
The most dramatic extension of the range of coconut mite in recent years occurred in the late 1990s, when it was found for the first time on coconuts in Tanzania (East Africa), India, and Sri Lanka.
Curiously, the coconut mite has not been reported in the South Pacific Region, which is the original home of the coconut palm.
Description
The adult female coconut mite, which is the largest stage, is 205 to 255 µm long and 36 to 52 µm wide. These minute arthropods cannot be seen distinctly with the naked eye. Massive colonies of the mites and individual mites can be detected with difficulty with a 10X hand lens. At this magnification, the colonies appear as vague silvery patches. Individual coconut mites appear small even when viewed under standard stereoscopic microscopes. Like eriophyid mites in general, they are elongate and possess two pairs of legs, instead of four pairs as is typical of mites of most families. They are white and translucent.
Coconut mite infestations are generally diagnosed by the appearance of their damage, confirmed by finding specimens of the mite on the fruits. Positive identification of the mite can be made by a specialist examining specimens mounted on slides under a compound microscope.
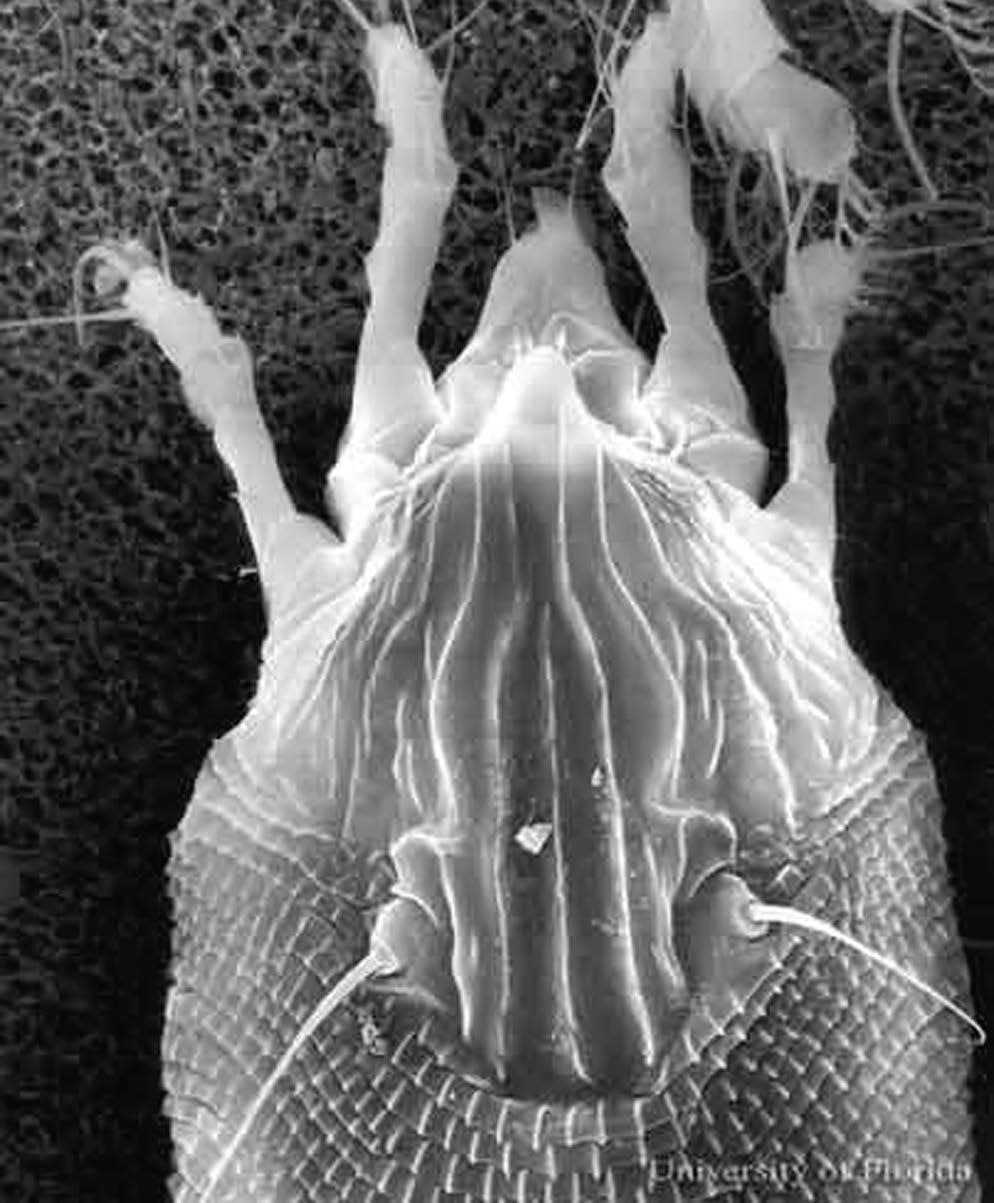
Credit: Greg Erdos, University of Florida. Used with permission of CABI Publications
Biology and Ecology
The mites infest the abaxial (lower) surfaces of the perianth and that part of the fruit surface that is covered by the perianth. They are able to penetrate between the tepals of the perianth and fruit surface a month after the fruit begins development; prior to this the tepals are too tightly appressed to allow entry of the mites. Presumably, a population on a fruit is initiated by one or more fertilized females usually from either infested fruits on the same or nearby plants. The mites feed by piercing the superficial plant tissue to access juices, which they then imbibe. A coconut mite develops from egg to adult in 10 days, thus populations beneath the perianth of a coconut fruit build up rapidly, often producing thousands of mites in each of several aggregations on the same fruit. Massive populations of coconut mites may be present among the tepals and on the fruit surface beneath the perianth until about the sixth month of the coconut's development, after which populations decline. Coconuts mature in about 12 months, at which time few if any coconut mites are present, even in coconuts with extensive damage by these mites.
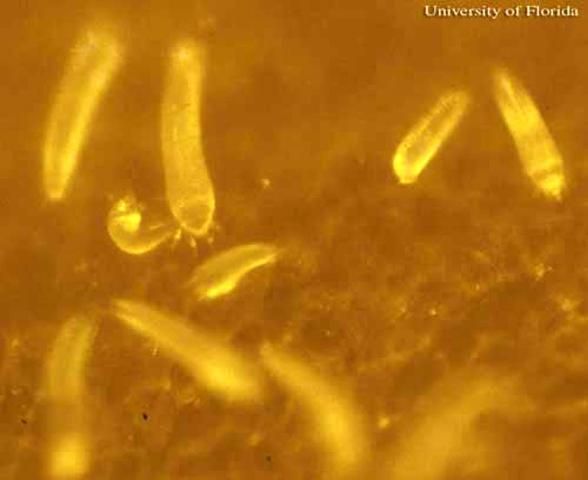
Credit: J. V. DeFilippis, University of Florida

Credit: F.W. Howard, University of Florida
Coconut mites probably disperse from one palm to the other on air currents, or by phoresy (e.g., carried on insects or birds that visit palm flowers). Where coconut palm plantings are dense, mites possibly crawl from the foliage of one palm to that of an adjacent palm and ultimately arrive on a fruit. Their inefficient host-finding capabilities seem to be compensated for by a high reproductive rate.
In Florida, coconut mite infestations are generally more prevalent on the Florida Keys than on the Florida mainland. For example, in a survey conducted in 1986–1987, 98% of the coconuts on some sites on the Keys were infested, while 0 to 8% of the coconut palms were infested on most sites examined within the range of coconut palms on the mainland. The environment of the Keys is apparently more suitable to the coconut mite than that of the mainland, but for reasons that remain elusive. The Keys receive about half the annual precipitation than the southern Florida mainland, and indeed some reports indicate that the coconut mite is most damaging to coconuts growing in relatively dry regions, and more damaging during the dry season in tropical areas with pronounced wet and dry seasons. However, observations of some researchers refute this; studies conducted at Bahia Honda in the Keys and at Añasco, Puerto Rico, revealed no association between seasonal rainfall patterns and coconut mite populations.
Coconut mites can undoubtedly spread to new host palms more easily if the palms are in close proximity, and there seems to be a positive correlation between planting density and the percentage of coconut palms infested with coconut mite.
Hosts
Coconut palm appears to be virtually the only host of coconut mite, although there is an isolated confirmed record from Brazil on the fruit of Lytocaryum weddellianmum (H. A. Wendland), a palm native to South America that is loosely related to coconut.
Coconut palm varieties differ in their susceptibility to coconut mite. Almost all varieties probably have some level of susceptibility.

Credit: F.W. Howard, University of Florida
Damage and Economic Importance
The meristematic zone from which the growing coconut fruit expands is a circular whitish area covered by the perianth. The young fruits of about 2.5–3.0 cm (~1–1.2 in) in diameter develop to the mature coconut of up to 25 cm (9.8 in) during the period of about one year. As the damaged surface expands from beneath the perianth and becomes exposed to air, it becomes suberized, that is, develops a brown cork-like surface with deep fissures. If intense mite feeding is concentrated on one side of the fruit meristem, growth of the fruit may be uneven, resulting in a distorted coconut. Highly severe damage results in stunting of the fruits.
Copra, a main product of the coconut industry, is the white kernel, or coconut "meat" after it is dried. In one study, coconut mite damage was found to cause a loss of up to 30% of the copra. Other researchers have reported a less serious impact on copra production. In many tropical countries, coconut water is a principal product. This is the clear liquid in the coconut that serves as a beverage and is sometimes erroneously called "coconut milk." (In the coconut industry, the latter term applies to the paste made by grinding the kernel.) An example of the commercial importance of coconut water is that in Puerto Rico about 10 million fresh coconuts are sold each year for coconut water. Data is not available on the possible impact of the coconut mite on the production of coconut water, but this product is generally marketed locally in fresh coconuts, and the unappealing appearance of mite-damaged coconuts has been shown to adversely affect sales. This interferes with the livelihood of many individuals.
A favorite motif in advertising tourist destinations in Florida and the Caribbean as well as other tropical locals is a view of a beach with gracefully swaying coconut palms stretching towards the sea. Additionally, coconut palms find a place in many designed landscapes of resort areas in these regions and are treasured by many homeowners. Damage by coconut mite is not highly noticeable from a distance, thus it often has no significant impact on the aesthetic appearance of palms on beaches and in many landscape situations. As a pest of ornamental plants, coconut mite is most important to homeowners or managers of areas where palms are seen up close, such as in the landscaping around hotel swimming pools.

Credit: F. W. Howard, University of Florida
Detection
An early-stage infestation of a young coconut by coconut mites is often detectable as a small, pale triangular area extending distally on the fruit surface from beneath the perianth. In other cases, a broader pale zone extends from the perianth. The pale areas turn brown in a matter of days. As infested coconuts develop, the damaged area continues to extend from beneath the perianth, eventually covering a large portion of the surface. In older damage, the affected surface is suberized (cork-like), with deep longitudinal fissures that may be intersected by horizontal cracks.

Credit: J. V. DeFilippis, University of Florida

Credit: J. V. DeFilippis, University of Florida
Prior to maturing, coconuts are green, yellow, bronze, apricot color, or a blend of these colors, depending on variety. Mature coconut fruits (i.e., of about 12 months of development) turn brown naturally. Thus, the dark brown color of advanced damage of coconut mite is most noticeable before the fruit has fully matured. Coconut mite damage can be spotted at a distance, but the diagnosis must be confirmed by closer examination. Browning of coconuts can be caused by various factors other than coconut mites, including various forms of mechanical damage. For example, a petiole constantly rubbing against a coconut in winds can cause browning over the affected area of the surface. A smooth brown surface may be the result of recent damage due to cold (e.g., after abnormally cold periods during winter in Florida).
In Florida and Puerto Rico, a second mite, Tarsonemus sp. (Acari: Tarsonemidae), causes damage similar in appearance to early coconut mite damage, but is rare. This mite occurs in populations of not more than a few hundred individuals per coconut and has been seen only on young coconuts. In a study involving a large number of coconuts in Florida that had damage attributable to mites feeding beneath the perianth, 99% were found to be infested with coconut mite, and only 1% with Tarsonemus sp.
Management
Predatory mites found beneath the coconut perianth in Florida and observed to prey on coconut mites include Amblyseius largoensis Muma, Neoseiulus mumai Denmark, and Neoseiulus paspalivorus DeLeon. In Puerto Rico, Bdella distincta Baker and Bablock preyed on coconut mite and on Steneotarsonemus furcatus (DeLeon) in the same habitat. In both of these localities, however, coconut mite infestations were heavy, implying that the effects of these predators were insignificant.
The fungus, Hirsutella thomsonii (Fisher), which is widely distributed and known to attack various species of mites, has been isolated from coconut mites in various countries, as has Hirsutella nodulosa Petch in Cuba. Control of several species of mites with fungus has been developed and applied, but success has often depended greatly on environmental conditions. In general, these efforts have been most successful under humid conditions favoring the development of the fungi.
Since coconut mites are almost microscopic and pass almost all of their life cycle in a cryptic habitat, it appears possible that in some regions the mite may be present at undetected levels. If such regions could be identified, they could be potential sources of effective natural enemies of the coconut mite.
A simple mechanical form of control practiced by some farmers is to prune all of the coconuts in all stages of development. This is said to eliminate coconut mites at least temporarily, but obviously causes a disruption in production. The method may be a useful in situations such as in landscapes with high tourist activity, where coconuts are periodically pruned to prevent injury caused by falling coconuts.

Credit: F. W. Howard, University of Florida
Field observations have indicated that at least in some situations there appears to be an inverse relationship between water available to the palms and damage levels of coconut mite. Other observations indicate that increased nutrient availability results in faster growth of coconuts so that they incur less coconut mite damage or tolerate it better. However, in other studies, increased nutrients seemed to increase the level of mite attack. Much research remains to be done to provide a basis for economically feasible cultural control of the coconut mite.
The coconut varieties most common in Florida and the Caribbean, viz., 'Jamaica Tall', 'Panama Tall', 'Malayan Golden Dwarf', 'Malayan Yellow Dwarf', and 'Malayan Green Dwarf' are all highly susceptible to coconut mite. Some observers have reported that certain varieties of coconut in some countries appear to be resistant to coconut mite. An apparently resistant Cambodian variety was reported on a research station in Africa. It was suggested that the very round shape of the fruit of this variety perhaps resulted in a tight perianth that excluded coconut mites. However, we have observed extensive damage of coconut mites on round-fruited coconut varieties in Florida and the Caribbean.
Acaricides have been tested for control of the coconut mite, and some have been shown to kill the mites. However, most chemicals applied topically had to be repeated often and indefinitely to maintain control. Systemic acaricides might persist longer in the plant, but such chemicals could result in residues in the fruits, and coconuts are harvested throughout the year. Chemical control is perhaps the least viable option for control of coconut mite.
Selected References
Fernando LCP, Moraes GJ, Wickramananda IR. 2002. Proceedings of the International Workshop on Coconut Mite (Aceria guerreronis), Sri Lanka, 6–8 January 2000. Coconut Research Institute, Sri Lanka. 117 pp.
Howard FW, Abreu-Rodriquz E, Denmark HA 1990. Geographical and seasonal distribution of the coconut mite, Aceria guerreronis (Acari: Eriophyidae), in Puerto Rico and Florida, USA. Journal of Agriculture, University of Puerto Rico 74: 237–251.
Howard FW, Moore D, Giblin-Davis R, Abad R. 2001. Insects on Palms. CABI Publications, Wallingford, UK.
Moore D. 2000. Non-chemical control of Aceria guerreronis on coconuts. Biocontrol News and Information, 21, 83N–87N.
Moore D, Howard FW. 1996. Coconuts. In Eriophyid Mites—Their Biology, Natural Enemies, and Control. Lindquist EE, Sabelis MW, Bruin J. (eds.). Elsevier Science BV. pp. 561–569.