This fact sheet is one of a series titled Reclaimed Water Use in the Landscape addressing various issues related to use and management of reclaimed water in urban landscapes.
Introduction and Purpose
The use of reclaimed water provides a way of recycling and reusing water, which reduces our consumption of potable water and ensures conservation of freshwater resources. Florida is a national leader in the use of reclaimed water. Approximately 673 million gallons per day (mgd) of reclaimed water was used for beneficial purposes in Florida in 2009 (FDEP 2010). This reuse reduced the use of nearly 127 billion gallons of potable water.
As shown in Figure 1, 56 percent of Florida's reclaimed water is used to irrigate public access areas like lawns, parks, schools, and golf courses. Another 11 percent is used to irrigate agricultural land, including edible crops such as citrus, vegetables, and berries. With more than two-thirds of the state's reclaimed water being applied to public lands and crops, knowing about the reclaimed water constituents and their impacts on landscape plants and human and ecosystem health is critical for proper management of this resource.
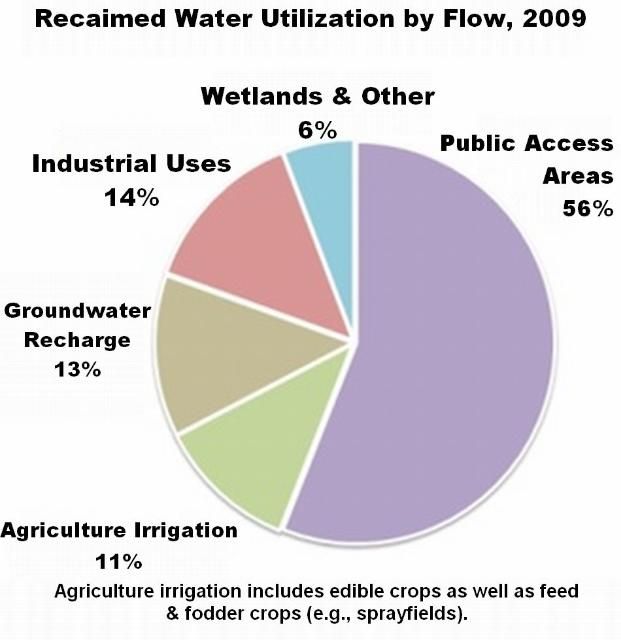
Credit: FDEP (2010)
The purpose of this publication is to provide information to wastewater operators, state agency personnel, Extension agents, homeowners, and landscapers on particular constituents of concern found in reclaimed water.
We divided constituents of concern into two broad categories for discussion purposes:
-
Constituents of concern primarily for the maintenance of landscape plants and turfgrass. These constituents are listed in Tables 1A and 1B and include water quality parameters that are routinely analyzed for irrigation water as well as several trace elements.
-
Constituents of concern primarily for human and ecosystem health. These constituents are listed in Table 2 and are physical, biological, and chemical factors that may affect human health or ecosystem functioning. Table 2 also identifies U.S. Environmental Protection Agency (EPA) guidelines for the acceptable concentrations of selected constituents.
Constituents of Concern for Landscape Plants and Turfgrass
For landscape plants and turfgrass, salts and nutrients are the most common constituents of concern in reclaimed water, and both are discussed below in more detail. Other minor constituents of concern in the landscape are summarized in Tables 1A and 1B.
Salts. The most problematic constituents of reclaimed water are total dissolved salts. These may occur naturally in water but can also enter the wastewater stream from domestic water use—which is why reclaimed water may contain sufficient salts to damage landscape plants and grasses without proper water management. Reclaimed water containing high amounts of salts can adversely affect plants in several ways, including the following:
-
reduced seed germination
-
reduced initial rooting
-
foliar damage
-
physiological drought—caused when salts attract water molecules and reduce water availability to plant roots
Plants differ in their sensitivity to salinity, but all plants have a maximum salt tolerance. In Florida, most turfgrasses have high to moderate salt tolerances. However, some Florida landscape plants have low salt tolerances. Figure 2 shows how chloride leaf-tip burn on ornamental plants can result from irrigation with reclaimed water exceeding the salt tolerance of that species. Figure 3 contrasts the effect of overhead and drip irrigation with reclaimed water on plant parts. Two Florida ornamentals especially susceptible to salt damage are crape myrtles and azaleas. Indications of salt problems for these plants include reddish-brown discoloration of the leaf tips and leaf drop. See https://edis.ifas.ufl.edu/EP012 for a list of common Florida landscape plants and their salt tolerances. One of the best cultural practices for landscaping is to have the right plant in the right place, so be sure to grow salt-tolerant plants if you are concerned about salts in reclaimed water. Also, check with your reclaimed water provider if you suspect that your irrigation water has high levels of salts. Figure 4 shows premature defoliation of leaves in a reclaimed water-irrigated site in Toronto, Canada.

Credit: Duncan et al. (2009)

Credit: Duncan et al. (2009)

Credit: Duncan et al. (2009)

Credit: Duncan et al. (2009)
Salts may also build up in the soil. A buildup of sodium (Na) in soils is especially problematic; it adversely affects soil structure and causes surface crusting of soil, low water permeability into the soil, and micronutrient deficiencies. Figure 5 shows salt-crusted soil on a golf fairway that was irrigated with moderately saline reclaimed water in southern California. Salt buildup in Florida soils is less common than in arid areas because our high rainfall levels leach sodium and other salts from the soil. However, we may have high salt accumulations in areas that are influenced by seawater via seawater intrusion in well waters.
The salt ions bicarbonate (HCO3) and carbonate (CO3) are of concern because they may combine with calcium (Ca) and magnesium (Mg) ions to form insoluble compounds. The formation of Ca and Mg compounds leads to a loss of Ca and Mg from the soil. When Ca and Mg are lost from the soil, the Na ion then gains preferential status and may accumulate, leading to the problems with sodium described above. You may have excessive levels of bicarbonate and carbonate in your reclaimed water if you see a white chalky precipitate building up on your sprinkler heads.

Credit: Duncan et al. (2009)
Nutrients. Even after advanced treatment at a wastewater treatment plant, almost all reclaimed water contains detectable amounts of the nutrients, nitrogen and phosphorus. If the reclaimed water is used for irrigation, it can be assumed that these nutrients may partially supply plant nutritional demand; no research yet is available to support the fact that reclaimed water provides nutrients to plants. When using reclaimed water for irrigation, be sure to test regularly for salt and nutrient levels and to incorporate the results in your landscape fertility plan. You may also be able to contact your local reclaimed water provider for information on nitrogen and phosphorus levels in your irrigation water.
See Tables 1A and 1B for more comments about constituents of concern for landscape plants and turfgrass. These tables also show desired or recommended values for many of the constituents, as well as advice for homeowners and landscapers on how to address the presence of the constituents in their irrigation water.
Constituents of Concern for Human and Ecosystem Health
Some constituents of reclaimed water are of concern because of how they could affect human or ecosystem health. These are divided into biological, physical, and chemical factors. Factors within each of these categories are summarized in Table 2. Some of the most common factors are also discussed below.
Biological Factors. Most living organisms that affect reclaimed water are microorganisms. They include bacteria, viruses, and protozoan, as well as some parasitic worms. Not all organisms found in reclaimed water will be human pathogens; some will be harmless and others will affect only certain plants or animals. The U.S. EPA (2004) has stringent guidelines for reclaimed water treatment to keep biological factors to a safe minimum. In Florida, harmful organisms have either been found to be present in less than infective doses (i.e., below levels that will make humans sick) or, in the case of viruses, not harmful to humans.
At all wastewater treatment plants, water is routinely tested for fecal coliform. Fecal coliforms are a subgroup of bacteria found in the intestine of warm-blooded animals and humans. Thus, they are indicators of human or animal wastes in the water source and can be a cause of disease if the water is consumed. Though the level of fecal coliform in reclaimed water is often low, you should avoid using reclaimed water for drinking or sanitary purposes. Your water provider can provide up-to-date information on test results for biological factors in your local reclaimed water.
Physical Factors. Total suspended solids (TSS) may be organic (plant parts, algae, bacteria), inorganic (sand grains, silt, clay), or immiscible liquids (grease, oils). If present at high levels, they interfere with water disinfection but are usually effectively filtered out of reclaimed water as it leaves the wastewater treatment plant. The U.S. EPA requires TSS levels to be maintained between 5 to 30 milligrams per liter (mg/L) for reclaimed water. Keeping TSS to this minimum level ensures that reclaimed water maintains a high level of disinfection. TSS levels are not a problem that most homeowners and landscapers will experience, as the treatment plant will ensure that EPA standards are being met.
Color and odor are two other physical parameters that can affect reclaimed water. See Table 3 for several potential causes of discoloration in reclaimed water.
The only odor associated with most reclaimed water is a slight chlorine smell from the disinfection process. It is not harmful to the landscape or public health. In rare occasions, odor in reclaimed water may be a sign of decaying organic matter. Your reclaimed water provider will have information about organic matter content, since this parameter is regularly monitored at the wastewater treatment plant. Organic matter is also discussed below.
Chemical Factors. One of the most common chemical concerns in reclaimed water is organic matter content. Organic matter in reclaimed water can be a problem for several reasons, including impacts on human health and reduced disinfection effectiveness. Organic matter content in water can be assessed by:
-
Biochemical oxygen demand (BOD), a measure of the oxygen required for decomposition of organic matter by aerobic bacteria.
-
Chemical oxygen demand (COD), a measure of the oxygen used during decomposition (oxidation) of organic matter.
Both of the above parameters are indicators of the organic quality of the water. The higher their values, the more organic matter present in the water. When values exceed those recommended in Table 2, the wastewater treatment plant will investigate the water for possible organic pollution.
Pesticides are another possible chemical constituent of reclaimed water. Pesticides may be problematic if they enter runoff or adversely affect the plants that are irrigated. Most reclaimed water samples are not routinely analyzed for pesticides. If you are concerned about pesticides in your reclaimed water, see if your provider can order a screening for pesticides that are common in your area.
Summary
Reclaimed water may contain numerous constituents of concern. The most important of these is total soluble salts. Salts can harm plants or build up in soil and cause problems such as loss of structure, salt crusting, and physiological drought. Other constituents of concern include nutrients, metals, and various biological, physical, and chemical factors. See Toor and Lusk (2011) What's in Reclaimed Water and Where Does It Go? at https://edis.ifas.ufl.edu/ss542 for more information on constituents of concern and their behavior in the environment. This publication has commented on what these constituents of concern are and has provided, where possible, recommended guidelines for their concentrations in reclaimed water.
The levels at which constituents of concern are found in reclaimed water can only be controlled by the provider. If users are concerned about excessive levels of any constituent, they should request to see water quality data from their provider. Homeowners and others who rely on reclaimed water for irrigation can avoid most problems by growing the right plants in the right places and by irrigating only when the landscape needs water. Many constituents of concern for public health are strictly monitored by wastewater treatment plants, but some others are not routinely analyzed or do not have concentration guidelines. For this reason, users should take care that reclaimed water is not consumed or used for sanitary purposes. Also, proper irrigation management, including only irrigating when necessary and controlling how much water is applied, will help ensure safe and reliable use of reclaimed water for both people and wildlife. See https://edis.ifas.ufl.edu/ep110 for information about how often you should irrigate Florida lawns and gardens.
References
Duncan, R.R., R. Carrow, and M. T. Huck. 2009. Turfgrass and Landscape Irrigation Water Quality: Assessment and Management. CRC Press, Boca Raton, FL.
FDEP. 2010. Florida's Reuse Activities. http://www.dep.state.fl.us/water/reuse/activity.htm. (accessed 01/04/2011).
US EPA. 2004. Guidelines for Water Reuse. US EPA, Office of Water, Washington, D. C.
Westcot, D. W, and R. S. Ayers. 1985. Irrigation water quality criteria. In G. S. Pettygrove and T. Asano (Eds.). Irrigation with Reclaimed Municipal Wastewater—A Guidance Manual. Lewis Publ., Chelsea, MI.
Tables
Reclaimed water constituents of concern for landscape plants and turfgrass: parameters routinely analyzed in irrigation water. Source: Duncan et al. (2009).(mg/L is milligrams per liter).
Reclaimed water constituents of concern for landscape plants and turfgrass: elements not normally analyzed in irrigation water. Source: Duncan et al. (2009). (mg/L is milligrams per liter.)
Reclaimed water constituents of concern for human and ecosystem health. (mg/L is milligrams per liter.)


