Intended for Extension agents, this publication highlights the importance of sulfur in row and vegetable crop production systems in Florida, such as its use in pesticides and fertilizers. Additionally, the publication provides insights into the perspective of the Environmental Protection Agency (EPA) on sulfur in naturally occurring aquatic systems. This publication's target audience includes agricultural producers, Extension agents, crop consultants, representatives of the fertilizer industry, state and local agencies, students, instructors, researchers, and interested Florida citizens.
Introduction
Sulfur (S) accounts for approximately 2.9% of the earth’s mass, making this element the fifth most abundant element on earth after iron (32.1%), oxygen (30.1%), silicon (15.1%), and magnesium (13.9%). The forms of sulfur in nature are elemental sulfur, sulfide, and sulfate minerals.
Sulfur is the fourth most important macronutrient after nitrogen, phosphorus, and potassium for plant nutrition. Sulfur can be found in plants, foods, and water. Plant leaves require S for chlorophyll formation; and most protein and enzymes required for plant metabolic activities consist of the element. The shoots take in the sulfur gas (SO2) available in the air. De Kok and company (2005) identified sulfur's role as the vital component of amino acids, viz. cystine, cysteine, and methionine. The sulfur uptake across major crops in agriculture is shown in Figure 1.
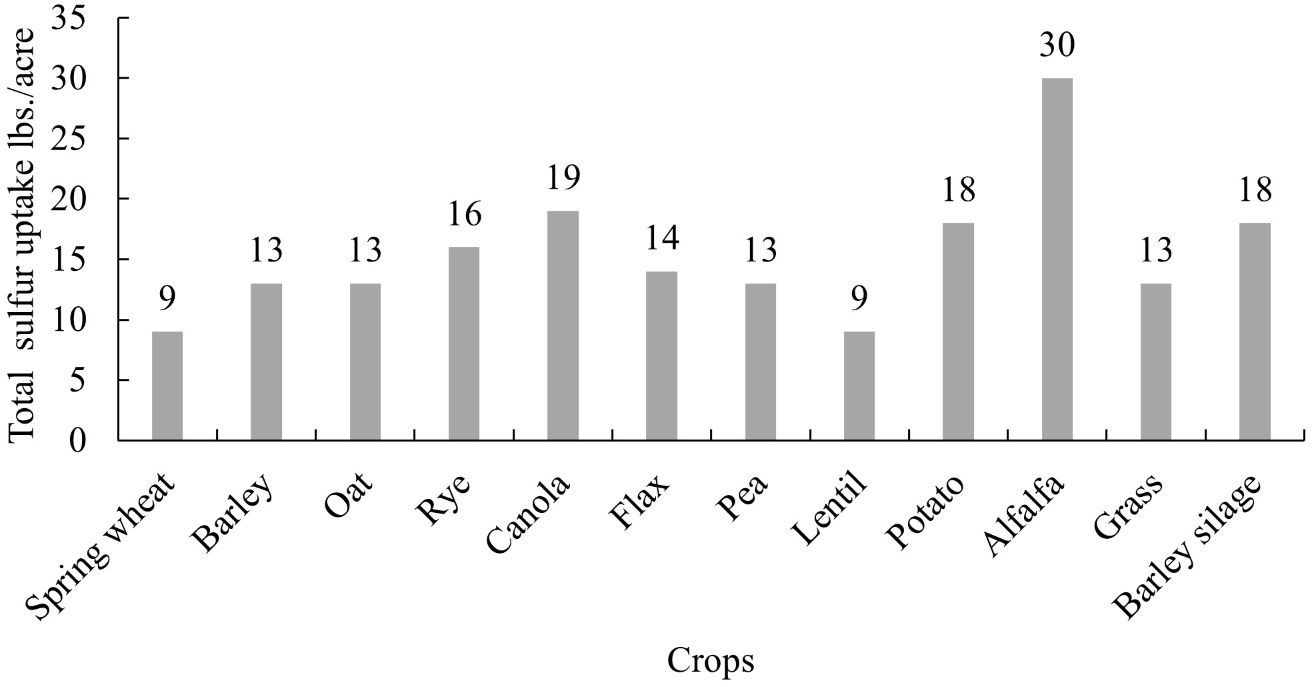
Credit: Government of Saskatchewan (2023)
Easily available in grocery stores, dilute sulfuric acid is often used for house cleaning and unblocking sewer pipes. Sulfuric acid is also used in many chemical analyses. Recently, south Florida has found an effective use of dilute sulfuric acid through irrigation to reduce soil pH as their soils are naturally alkaline (Longstroth 2012).
Role of Sulfur in Agriculture
Sulfur is an essential nutrient for plants, and its deficiency restricts plant growth and development. Figure 1 represents the sulfur uptake by major crops in the United States. In Florida, potato is the major crop (Figure 1) with an uptake of approximately 18 lb./acre of sulfur. Due to the decline in the environmental deposition of sulfur (Figures 2 and 3), the use of sulfur in agriculture has increased exponentially (Figure 4). The sulfate ion deposition in 1982–1984 was approximately 21 lb./acre. This figure was reduced to less than 7 lb./acre by 2012 and would stay so until 2014. During the same period, sulfur application increased across different crop production systems (Figure 4).
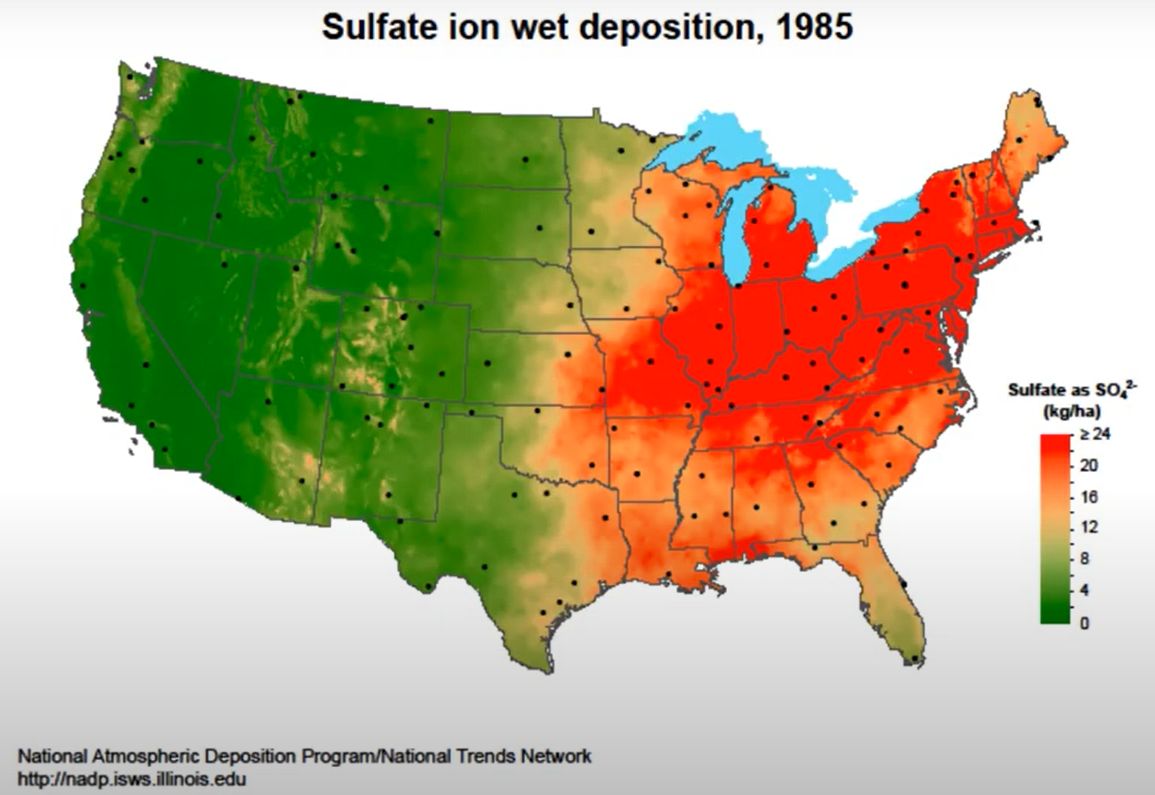
Credit: (2018)
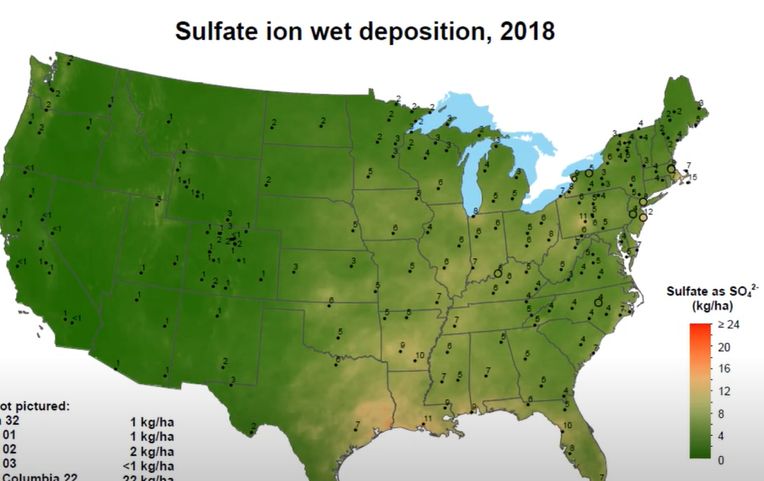
Credit: (2018)
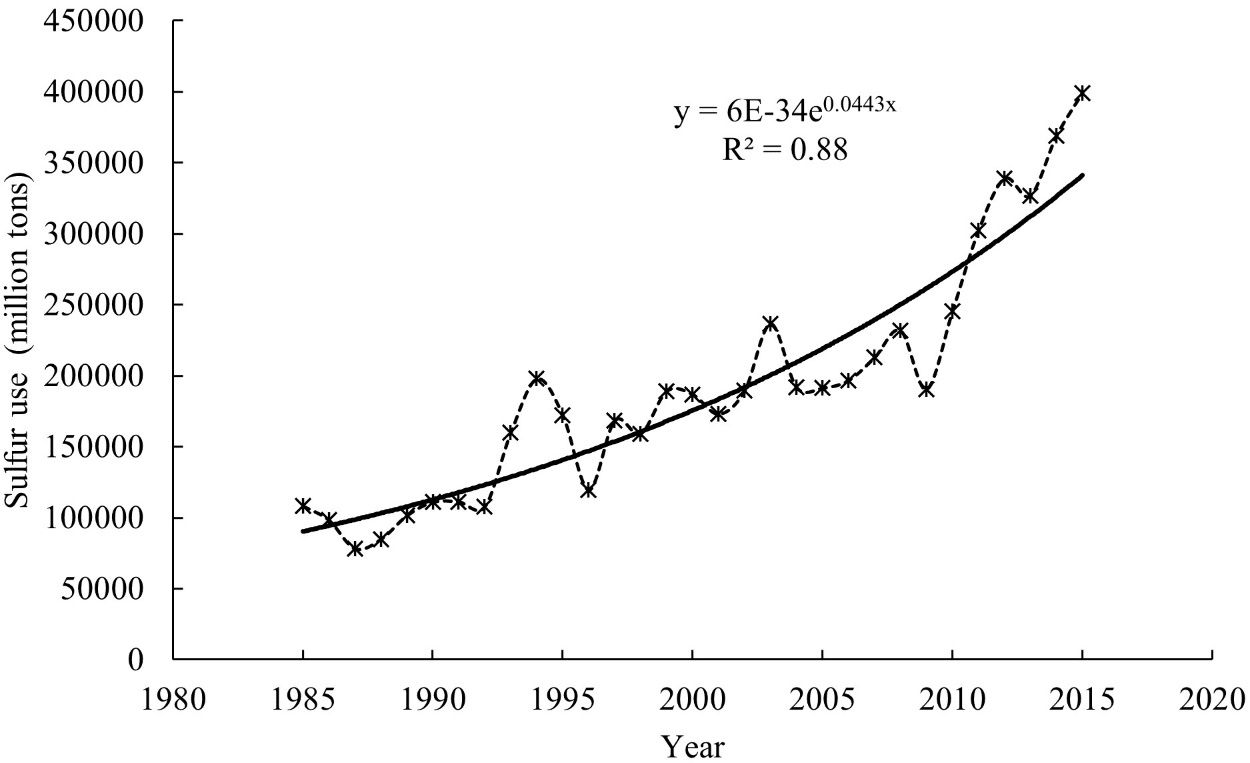
Credit: Hinckley (2022)
Sulfur is not mobile inside the plant; therefore, its deficiency appears on young leaves first. Both low and high levels of sulfur can negatively impact yield. Sulfur deficiency frequently occurs in sandy, well-drained soils with low organic content. In the United States, corn is particularly susceptible to sulfur deficiency (Figure 5). Sulfur deficiency could lead to poor nitrogen metabolism, stunted growth, low protein synthesis, and dwarfness (Divito et al. 2015). Potential visual symptoms could include the yellowing of young leaves, i.e., chlorosis, and necrosis in short slender stalks (Vidyalakshmi et al. 2009). Sulfur deficiency also affects the nitrogen fixation by the microbes (Saha et al. 2018; Chaudhary et al. 2022). Large amounts of sulfur are required by oilseed crops such as peanuts (Zhao et al. 1999).

Credit: Dr. Ron Hieniger, NC State Extension
Sulfur protects plants (Zhao et al. 2008): its volatile nature repels pathogens and herbivores. Klikocka et al. (2005) found that lower soil pH due to sulfur-based fertilizers increased potato plants' resistance to Streptomyces scabies and reduced the likelihood of Rhizoctonia solani infections.
Common Sulfur Fertilizers Used in Agriculture
Sulfur is used as fertilizer for many crops to replenish soil sulfur deficiency. Thanks to its slow, controlled release, S can also coat nitrogen fertilizer throughout the crop growth cycle to avoid potential leaching. Several innovative fertilizer technologies use sulfur as coating material.
Sulfur fertilizers can be categorized into 4 different groups:
- fertilizers that contain sulfate,
- fertilizers that contain elemental sulfur,
- fertilizers that contain both sulfate and elemental sulfur, and
- liquid sulfur fertilizers.
Sulfate-containing fertilizers are most common among farmers to provide sulfur to plants through soil application. This application provides multi-nutrient fertilizer in the form of SO4-2 that is immediately available to plants after application. The popular sources of this type of fertilizer are ammonium sulfate, calcium sulfate, potassium sulfate, and magnesium sulfate.
The elemental sulfur-based fertilizers are concentrated sulfur carriers. They can reduce the soil pH and could be applied as an additive to nitrogen, phosphorus, or potassium fertilizers.
Liquid application of sulfur has gained importance because of the low solubility of sulfate fertilizers.
S Interaction with N and P
Sulfur interacts with nitrogen (N) and phosphorus (P) in the soil and plant. Because S and N share three crucial amino acids (cysteine, cystine, and methionine), their interaction affects the plant’s ability to uptake S from the soil. When a plant is deficient in N, the S moves with the N into the plant, resulting in a reduced S deficiency. However, when N is sufficient, the S cannot move into the plant, resulting in S deficiency. For example, optimum yield and quality in beans require one part S for every 12 to 15 parts N (Stewart and Porter 1969). (The required ratio for optimum growth changes with each crop type.) Any change in soil and plants’ optimum nutrient ratio reduces N efficiency, thereby allowing N to be lost to the environment. Continued studies on S’s and N’s interaction in row and vegetable crops are necessary to determine application rates for optimized nutrient efficiency and the best management strategies to maintain a proper balance of these elements.
The literature suggests that S and P negatively affect each other’s efficiency (Aulakh and Pasricha 1977). Thus, it is expected that higher S application rates reduce plants’ P uptake more so than lower S application rates. With higher applications, less P would be removed from the soil by the crop, leading to a possible lower P efficiency. Elevated soil phosphorus (P) levels in Florida indicate abundant availability (Liu et al. 2015). Since S and P ratios are still unknown for Florida soils, it is necessary to study their interaction to determine optimum application rates for optimized nutrient use efficiency.
Sulfur Use in Insecticides
Several pests—including insects, fungus, and even rodents—may be controlled using sulfur. In the year 1920, the Environmental Protection Agency granted registration for the use of sulfur in pesticides (EPA 2015). In the United States, there are more than 200 different insecticides available for purchase that include sulfur (EPA 2017). Some of them are used in the manufacture of organic foods, while others are used as pesticides, soil additives, or fertilizers (EPA 2013a).
Sulfur in the Environment: Agricultural Uses and Health Risks
Sulfur is a vital element for humans, animals, and plants. It can be found in various foods, such as onions and eggs, and is a regular part of our daily diet (EPA 2013). Once consumed, sulfur is reduced to hydrogen sulfide in the digestive tract, absorbed, and integrated into tissues and cartilage within the human body. As a result, exposure to sulfur is assumed to be both common and anticipated based on a balanced diet. The EPA has concluded that sulfur does not cause cancer, gene alteration, or gene damage (EPA 2013b). A person can be exposed to sulfur while applying sulfur dust, sprays, and other products through the skin, eyes, and breathing. It is important to read the product labels and follow proper protection measures. Prolonged exposure of the skin to sulfur may cause rashes and calluses.
Sulfur can kill fungi. However, the exact mode of sulfur as a fungicide is not well understood (Turner 2015). Sulfur is believed to enter fungi cells and affect their respiration (William and Cooper 2004).
Environmental Impact
Sulfur does not negatively affect the environment as it is a naturally occurring element (EPA 2013a). Elemental sulfur is regularly used as fertilizer and applied as sulfate or as elemental sulfur in soil acidifiers. Sulfur does not dissolve well in water. Thus, any drift or runoff to the water body is highly unlikely to impact aquatic life. However, it may damage sulfur-sensitive plants in treated areas (EPA 2013a).
Conclusion
Sulfur is a naturally occurring element essential to plant growth, development, and, thus, optimum crop yield. Sulfur also affects N and P efficiencies; therefore, its status in the soil needs to be aligned with crop requirements. EPA has found S to be an unharmful nutrient to the environment and human life.
Additional Sources
Please visit these sites for more information on sulfur:
- https://pubmed.ncbi.nlm.nih.gov/11896744/
- https://www.sulphurinstitute.org/pub/?id=8c64bf34-bc30-5bd9-0719-f6de83f7e84
- https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-077501_16-Apr-91.pdf
- https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-077501_1-May-91.pdf
References
Aulakh, M. S., and N. S. Pasricha. 1977. “Interaction Effect of Sulfur and Phosphorus on Growth and Nutrient Content of Moong (Phaseolus aureus L.).” Plant Soil 47: 341–350. https://doi.org/10.1007/BF00011493
Chaudhary, S., R. Dhanker, K. Singh, B. Brar, and S. Goyal. 2022. “Characterization of Sulfur-Oxidizing Bacteria Isolated from Mustard (Brassica juncea L.) Rhizosphere having the Capability of Improving Sulfur and Nitrogen Uptake.” Journal of Applied Microbiology 133 (5): 2814–2825. https://doi.org/10.1111/jam.15742
De Kok, L. J., A. Castro, A. Koralewska, M. Durenkamp, F. S. Posthumus, C. E. E. Stuiver, L. Yang, and I. Stulen. 2005. “Pathways of Plant Sulfur Uptake and Metabolism: an Overview.” Landbauforschung Volkenrode 283: 5–13.
Divito, G. A., H. E. Echeverría, F. H. Andrade, and V. O. Sadras. 2015. “Diagnosis of S Deficiency in Soybean Crops: Performance of S and N:S Determination in Leaf, Shoot and Seed.” Field Crop Research 180 (2015): 167–175. https://doi.org/10.1016/j.fcr.2015.06.006
Government of Saskatchewan, Canada. 2023. “Sulfur Fertilization in Crop Production.” https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/crops-and-irrigation/soils-fertility-and-nutrients/sulphur-fertilization-in-crop-production#:~:text=Generally%2C%20plants%20require%20about%20a,crops%20(see%20Table%201)
Hinckley, E.S. Midwest U.S. Fertilizer Data, 1985–2015. V1. November 23, 2022. Distributed by Environmental Data Initiative. https://doi.org/10.6073/pasta/2868799978abc180ff22ebc8da880248
US Environmental Protection Agency, Office of Pesticide Programs, Environmental Fate and Effects Division, US Government Printing Office. Preliminary Environmental Fate and Ecological Risk Assessment (PEFERA) for the Registration Review of Sulfur. 2013a.
US Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division, US Government Printing Office. Summary of Human Health Risk Assessments (SHHRA) to Support Registration Review. 2013b.
US Environmental Protection Agency, Office of Pesticide Programs, Pesticide Re-evaluation Division, US Government Printing Office. Interim Registration Review Decision for Sulfur (IRRDS). 2015.
Klikocka, H., M. Cybulska, and A. Nowak. 2017. “Efficiency of Fertilization and Utilization of Nitrogen and Sulphur by Spring Wheat.” Polish Journal of Environmental Studies 26 (5): 2029–2036. https://doi.org/10.15244/pjoes/69942
Liu, G. D., E. H. Simonne, K. T. Morgan, and G. J. Hochmuth. 2015. “Soil and Fertilizer Management for Vegetable Production in Florida.” University of Florida, IFAS Extension, HS711. https://edis.ifas.ufl.edu/publication/CV101
Longstroth, M. 2012. “Soil Test Before You Plant Blueberries.” Michigan State University Extension. https://www.canr.msu.edu/news/soil_test_before_you_plant_blueberries
National Atmospheric Deposition Program. 2018. “Site NTN AB32: Build a Report.” Data from 1985 to 2018. http://nadp.slh.wisc.edu/NTN
National Pesticide Information Center (NPIC). 2017. “Sulfur.” NPIC Product Research Online (NPRO). Corvallis, OR.
Saha, B., S. Saha, P. D. Roy, D. Padhan, S. Pati, and G. C. Hazra. 2018. “Microbial Transformation of Sulfur: An Approach to Combat the Sulfur Deficiencies in Agricultural Soils.” In Role of Rhizospheric Microbes in Soil, edited by V. S. Meena. Singapore: Springer Nature. https://doi.org/10.1007/978-981-13-0044-8_3
Stewart, B. A., and L. K. Porter. 1969. “Nitrogen-Sulfur Relationships in Wheat (Triticum aestivum L.), Corn (Zea mays), and Beans (Phaseolus vulgaris).” Agronomy Journal 61 (2): 267–271. https://doi.org/10.2134/agronj1969.00021962006100020027x
Turner, J. A. 2017. The Pesticide Manual: a World Compendium. 17th ed. Hampshire: British Crop Protection Council. 1048–1049.
Vidyalakshmi, R., R. Paranthaman, and R. Bhakyaraj. 2009. “Sulfur Oxidizing Bacteria and Pulse Nutrition–a Review.” World Journal of Agricultural Sciences 5 (3): 270–278.
Williams, J. S., and R. M. Cooper. 2004. “The Oldest Fungicide and Newest Phytoalexin–a Reappraisal of the Fungitoxicity of Elemental Sulphur.” Plant Patholology 53 (3): 263–279. https://doi.org/10.1111/j.0032-0862.2004.01010.x
Zhao, F.J., M. T. Hawkesford, and S. P. McGrath. 1999. “Sulfur Assimilation and Effects on Yield and Quality of Wheat.” Journal of Cereal Science 30: 1–17. https://doi.org/10.1006/jcrs.1998.0241
Zhao, F. J., M. Tasuz, L. J. De Kok. 2008. “Role of Sulphur for Plant Production in Agricultural and Natural Ecosystems.” In Sulphur metabolism in Phototrophic Organisms, edited by Rüdiger et al., 425–443. http://dx.doi.org/10.1007/978-1-4020-6863-8_21