Introduction
Uterine diseases in dairy cows can be classified as puerperal metritis, clinical metritis, clinical endometritis, and subclinical endometritis (Sheldon et al. 2006). These diseases are highly prevalent in high-producing dairy cows and have been associated with decreased pregnancy per artificial insemination (AI), extended interval to pregnancy, increased culling, and economic losses (Bartlett et al. 1986; Sheldon and Dobson 2004; Gilbert et al. 2005). Metritis affects about 20.0% of lactating dairy cows, with the incidence ranging from 8% to > 40% in some farms (Curtis et al. 1985; Galvão et al. 2009a; Goshen and Shpigel 2006; Hammon et al. 2006; Huzzey et al. 2007). Clinical endometritis also affects about 20.0% of lactating dairy cows, with the prevalence ranging from 5.0% to > 30% in some herds (Galvão et al. 2009a; LeBlanc et al. 2002; McDougall et al. 2007). Subclinical endometritis is the most prevalent of all uterine diseases; it affects ~30% of lactating dairy cows, with the prevalence ranging from 11% to > 70% in some herds (Barlund et al. 2008; Galvão et al. 2009a; Gilbert et al. 2005; Hammon et al. 2006; Kasimanickam et al. 2004).
Retention of fetal membranes is a condition in which the cow fails to release the placenta 12 or 24 hours after calving. Although retention of fetal membranes is not a disease per se, many researchers have tried to treat (systemically or intrauterine) this condition because it is a major risk factor for metritis (Drillich et al. 2006; Goshen and Shpigel 2006; Risco and Hernandez 2003). Although treatment has been found to prevent metritis (Risco and Hernandez 2003), it has not been found to improve fertility or milk yield (Drillich et al. 2006; Goshen and Shpigel 2006; Risco and Hernandez 2003); therefore, it will not be emphasized in this paper. Pyometra is characterized by a pus-filled uterus in the presence of a corpus luteum (CL) and a closed cervix (Sheldon et al. 2006). Pyometra can be considered a subset of endometritis in which cows ovulate in the presence of a contaminated uterus. Common treatment is administration of prostaglandin F2α (PGF2α).
Identification
Metritis
Puerperal metritis is characterized by the presence of an abnormally enlarged uterus, a fetid watery red-brownish uterine discharge associated with signs of systemic illness, and fever (> 103°F) within 21 days in milk (DIM). Animals without systemic signs but with an enlarged uterus and a fetid uterine discharge within 21 DIM may be classified as having clinical metritis (Sheldon et al. 2006). Metritis is diagnosed by a complete physical examination of the cow including attitude, hydration status, rectal temperature, and palpation of the uterus per rectum to evaluate uterine discharge. Evaluation of rectal temperature should be performed before palpation per rectum. A Florida study (Benzaquen et al. 2007) observed that a high proportion (~60%) of cows did not have a fever (> 103°F) at the time puerperal metritis was diagnosed, indicating that this condition is not always accompanied by a fever. This finding suggests that diagnosis and treatment consideration for puerperal metritis should include the character of the uterine discharge (fetid or not) and the attitude of the cow, besides measurement of rectal temperature. Cows diagnosed with metritis without a fever were just as likely to later develop clinical endometritis as cows with metritis and a fever. This suggests that metritis without a fever might have the same negative effects on fertility as metritis with a fever (Benzaquen et al. 2007).
Cows diagnosed with metritis (puerperal or clinical) should be evaluated for concurrent metabolic or infectious diseases (ketosis, displaced abomasum, mastitis, pneumonia, etc.) since these conditions are associated (Curtis et al. 1985). Although not performed on a routine basis, vaginal examination can be performed to aid in diagnosis if a cow has a fever of unknown origin and no uterine discharge can be produced after palpation of the uterus per rectum. Care should be taken to wash the vulva with antiseptic solution (e.g., iodine scrub) and to use a clean, well-lubricated palpation sleeve (Williams et al. 2005). Dairies should have a clear standard operating procedure on when to evaluate cows for metritis and how to identify them. Metritis can occur at any time after calving, even after 21 DIM; however, most of the cases (~95%; 709/753) occur in the first 14 DIM with a peak around 5-7 DIM (Figure 1).
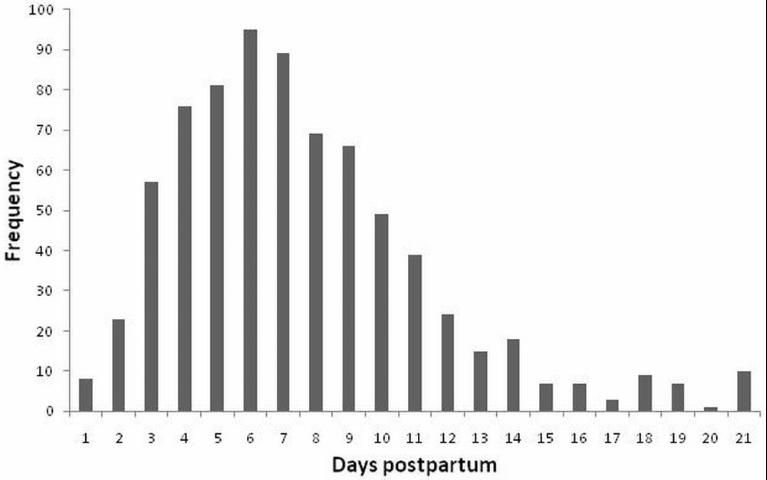
Because of this concentration in incidence of metritis in the first 14 DIM, and in an effort to target monitoring of cows, different strategies have been proposed by the pharmaceutical industry and by academia to diagnose and manage metritis. Pfizer (Pfizer Animal Health, New York, NY) devised what is called the 100-day contract for health and reproductive management. Part of that program includes daily monitoring of fresh cows in the first 10 DIM. Although monitoring cows in the first 10 DIM would be sufficient to diagnose most of the cows, a substantial proportion (~20%; 140/753) would be missed. At the University of Florida Dairy Research Unit, a combination of targeted monitoring of all cows at 4, 7, and 12 DIM in combination with physical examination of cows with milk deviation of more than 12% or failure to increase milk yield at least 4% (primiparous) or 7% (multiparous) per day in the first 20 DIM have proven very efficacious in diagnosing cows with metritis and metabolic diseases (ketosis and displaced abomasum). Although the system has proven effective, it requires individual daily milk weights. Others have targeted the first 13 (Benzaquen et al. 2007) or 14 DIM (Galvão et al. 2009a) for daily monitoring. Regardless of the monitoring regimen adopted, compliance with the protocol and skill of the evaluator are paramount to the success of the monitoring program.
Endometritis
Clinical endometritis is characterized by the presence of purulent (> 50%) uterine discharge after 21 DIM or mucopurulent (50% pus, 50% mucus) after 26 DIM (Sheldon et al. 2006). Clinical endometritis is usually diagnosed by evaluation of uterine discharge detected in the vagina with the aid of a speculum (LeBlanc et al. 2002), the Metricheck tool (McDougall et al. 2007), or a gloved hand (Williams et al. 2005). When using either one of these methods, care should be taken to clean the vulva, to avoid introduction of contaminants into the vagina, and to use lubrication. When using vaginoscopy, the speculum should be introduced into the vagina up to the level of the external os of the cervix, and inspection of the discharge is performed with the aid of a flashlight. When using the Metricheck tool (Metricheck, Simcro, New Zealand), the device should be introduced into the vagina up to the level of the external os of the cervix and the discharge should be scooped for evaluation after exteriorization of the device. When using a gloved hand, the hand should be introduced into the vagina up to the level of the external os of the cervix and the discharge should be scooped for evaluation after exteriorization of the hand.
In the absence of clinical endometritis, subclinical endometritis is defined by the presence of >18% neutrophils (PMN) in uterine cytology samples collected between 21 and 33 DIM or > 10% PMN between 34 and 47 DIM (Sheldon et al. 2006). Uterine cytology samples can be collected using the cytobrush (Kasimanickam et al. 2004) or the low-volume uterine lavage (Gilbert et al. 2005) technique. For the cytobrush, a Pap smear cytology brush is attached to a metal rod that is fitted through a metal pipe similar in diameter to an insemination pipette (Figure 2). The tool is protected with a plastic sheath protector during insertion into the vagina, and then is exposed for passing through the cervix. At the uterine body, the cytobrush is exposed and the body wall is pressed slightly against the cytobrush while the cytobrush is rolled two or three times. After that, the tool is exteriorized, and the cytobrush is smeared onto a glass slide and air dried before staining using Diff-Quick stain.

For the low-volume uterine lavage, an infusion pipette is protected with a sanitary chemise, which is punctured before the pipette is passed though the cervix. At any place in the uterus, 10-20 ml of sterile saline solution is infused, the uterus is then massaged and a portion (= 5 ml) is harvested. A folley catheter can also be used to perform a low volume lavage in a manner similar to embryo flushing (Galvão et al. 2009a). After collection, the sample needs to be centrifuged in a conventional or cytospin centrifuge. If using a conventional centrifuge, most of the supernatant needs to be discarded and one drop of the remaining fluid is smeared onto a glass slide. For the cytospin, ~150 µl of the collected sample is loaded in the cytospin container and centrifuged at 700 g for 5 min. Then, slides are air dried and stained using Diff-Quick. After staining, all cells, including epithelial cells but excluding erythrocytes, are counted under the microscope, and the proportion of PMN out of a total of 200 cells is calculated.
Endometritis has been diagnosed by detection of fluid in the uterus using ultrasonography (Kasimanickam et al. 2004); nonetheless, this method was found to be less sensitive than endometrial cytology (Barlund et al. 2008).
Treatment
Metritis
The most common method of treatment is either intrauterine (Galvão et al. 2009a; Goshen and Shpigel 2006; Kasimanickam et al. 2005; LeBlanc et al. 2002; Thurmond et al. 1993) or systemic (Chenault et al. 2004) antibiotic administration. Currently, in the United States, there is no approved antibiotic for intrauterine administration in dairy cows. There are only three approved antibiotics for systemic administration for treatment of metritis in dairy cows: ceftiofur hydrochloride (Excenel®, Pfizer Animal Health, Madison, NJ) and ceftiofur crystalline-free acid (Excede®, Pfizer Animal Health, Madison, NJ), which are broad-spectrum third-generation cephalosporins, and Liquamycin LA-200 (Pfizer Animal Health), a long-acting oxytetracycline. Because of the long withhold time for milk (4 days) and meat (28 days) for Liquamycin, Excenel® (no withold for milk and 3 days for meat) is the treatment of choice. Excede also has no withhold for milk but has a 13 day withhold for meat; therefore, its adoption is still uncertain. The advantage of Excede would be the treatment regimen; two doses 72 h apart compared to daily injection for 5 days for Excenel. The recommended dose for treatment of metritis in postpartum dairy cows using Excenel is 2.2 mg/kg (2 ml/100 lbs) of body weight intramuscularly. The recommended dose for Excede is 6.6 mg/kg (1.5 ml/100lbs) of body weight given in the middle third or the base of the ear (https://www.zoetisus.com/content/_assets/docs/dairy/Excede-Marketing-Package-Insert.pdf). It is recommended to alternate ears.
Although systemic administration of ceftiofur hydrochloride improves clinical signs of metritis (Chenault et al. 2004), the effects on fertility have not been evaluated. On the other hand, intrauterine treatment with 5 g chlortetracycline twice weekly for 2 weeks prevented the negative effects of metritis on fertility and milk yields in multiparous cows (Goshen and Shpigel 2006); however, this treatment is not approved in the United States and would lead to long milk withdrawals. Assuming that the treatment would cost $10 and milk would be discarded for 21 d, the overall cost would be $199 (60 lbs x 21 x $15.00 cwt = 189 + 10 = $199). Cows that received this treatment regimen produced 1,438 lbs more milk and conceived 29 d sooner; therefore, the return would be $273.70 ((1438/100 x 15) + (29 x $2 per additional day open)), and the net profit would be $74.70.
Endometritis
A formulation containing 500 mg of cephapirin benzathine in 19 g emulsifier (Metricure®, Intervet, Boxmeer, The Netherlands) is approved for treatment of clinical endometritis by intrauterine administration in Canada, Europe, New Zealand, Australia, and other countries around the world. Intrauterine infusion of Metricure® improved reproductive performance of cows with clinical endometritis (LeBlanc et al. 2002). In the same study, treatment with prostaglandin F2α (PGF2α) was found to be intermediate. A large clinical trial found that PGF2α did not improve fertility in cows with clinical endometritis (Dubuc et al. 2011). Treatment with Metricure® was also found to improve fertility in cows with a history of retained fetal membranes, stillbirths, or a vulval discharge after 13 DIM (McDougall 2001). Nonetheless, a formulation containing 125 mg of ceftiofur hydrochloride in 10 mL oil-based sterile suspension (Spectramast LC, Pfizer Animal Health, New York, NY) labeled for treatment of clinical mastitis was shown to reduce the bacterial contamination of dairy cows with clinical endometritis; however, it did not improve fertility (Galvão et al. 2009a).
Although there is no approved treatment for subclinical endometritis, Metricure® was found to improve reproductive performance of cows with subclinical endometritis (Kasimanickam et al. 2005). Interestingly, in that study, PGF2α had a similar beneficial effect (Kasimanickam et al. 2005). The benefit from PGF2α administration is believed to arise from induction of estrus in cows having a PGF2α-responsive corpus luteum; the estrus leads to physical expulsion of bacterial contaminants and inflammatory products as well as a possible improvement in the uterine defenses under low progesterone (Kasimanickam et al. 2005). It is generally agreed that a high-progesterone environment suppresses cervical mucus production, myometrial contractility, uterine-gland secretion, and the phagocytic activity of uterine neutrophils (Frank et al. 1983; Hussain 1989; Bondurant 1999), and is therefore permissive to uterine infection. PGF2α is not only luteolytic but also appears to have pro-inflammatory actions that might enhance neutrophil function (Lewis 2004). Because there is increased concern about bacterial acquisition of antibiotic resistance, PGF2α would provide an efficacious method of treatment of endometritis. Nonetheless, later studies found no beneficial effect of PGF2α for treatment of subclinical endometritis (Galvão et al. 2009b; Dubuc et al. 2011); therefore, the combined literature suggests that PGF2α is not an efficacious method to treat subclinical endometritis.
Conclusions
Uterine diseases are prevalent in high-producing dairy cows and require prompt diagnosis and treatment. Metritis can be successfully treated either by systemic or intrauterine antibiotic treatment. Ceftiofur hydrochloride (Excenel®) intramuscularly or cefitofur crystalline-free acid (Excede®) subcutaneously is effective in treating metritis, and oxytetracycline intrauterine is effective in abrogating the negative effects of metritis on milk yield and fertility. Intrauterine administration of cephapirin benzathine (Metricure®) is an effective treatment for clinical and subclinical endometritis, although not approved in the United States. Administration of PGF2α does not seem effective for the treatment of clinical or subclinical endometritis.
References
Barlund, C.S., T.D. Carruthers, C.L. Waldner, and C.W. Palmer. 2008. "A comparison of diagnostic techniques for postpartum endometritis in dairy cattle." Theriogenology 69: 714–23. https://doi.org/10.1016/j.theriogenology.2007.12.005
Bartlett P.C., J.H. Kirk, M.A. Wilke, J.B. Kaneene, and E.C. Mather. 1986. "Metritis complex in Michigan Holstein-Friesian cattle: incidence, descriptive epidemiology and estimated economic impact." Prev. Vet. Med. 4: 235–48. https://doi.org/10.1016/0167-5877(86)90026-7
Benzaquen, M.E., C.A. Risco, L.F. Archbald, P. Melendez, M.J. Thatcher, and W.W. Thatcher. 2007. "Rectal temperature, calving-related factors, and the incidence of puerperal metritis in postpartum dairy cows." J. Dairy Sci. 90: 2804–14. https://doi.org/10.3168/jds.2006-482
Bondurant, R.H. 1999. "Inflammation in the bovine female reproductive tract." J. Anim Sci. 77 (Suppl 2): 101–10. https://doi.org/10.2527/1999.77suppl_2101x
Chenault, J.R., J.F. McAllister, S.T. Chester Jr., K.J. Dame, F.M. Kausche, and E.J. Robb. 2004. "Efficacy of ceftiofur hydrochloride sterile suspension administered parenterally for the treatment of acute postpartum metritis in dairy cows." J. Am. Vet. Med. Assoc. 224: 1634–39. https://doi.org/10.2460/javma.2004.224.1634
Curtis, C.R., H.N. Erb, C.J. Sniffen, R.D. Smith, and D.S. Kronfeld. 1985. "Path analysis of dry period nutrition, postpartum metabolic and reproductive disorders, and mastitis in Holstein cows." J. Dairy Sci. 68: 2347–60. https://doi.org/10.3168/jds.S0022-0302(85)81109-7
Drillich, M., U. Reichert, M. Mahlstedt, and W. Heuwieser. 2006. "Comparison of two strategies for systemic antibiotic treatment of dairy cows with retained fetal membranes: preventive vs. selective treatment." J. Dairy Sci. 89: 1502–8. https://doi.org/10.3168/jds.S0022-0302(06)72217-2
Dubuc, J., T.F. Duffield, K.E. Leslie, J.S. Walton, and S.J. Leblanc. 2011. "Randomized clinical trial of antibiotic and prostaglandin treatments for uterine health and reproductive performance in dairy cows." J. Dairy Sci. 94: 1325–38. https://doi.org/10.3168/jds.2010-3757
Frank, T., K.L. Anderson, A.R. Smith, H.L. Whitmore, and B.K. Gustafsson. 1983. "Phagocytosis in the uterus: a review." Theriogenology 20: 103–10. https://doi.org/10.1016/0093-691X(83)90029-8
Galvão, K.N., L.F. Greco, J.M. Vilela, M.F. Sá Filho, and J.E.P. Santos. 2009a. "Effect of intrauterine infusion of ceftiofur on uterine health and fertility in dairy cows." J. Dairy Sci. 92: 1532–42. https://doi.org/10.3168/jds.2008-1615
Galvão, K.N., M. Frajblat, S.B. Brittin, W.R. Butler, C.L. Guard, and R.O. Gilbert. 2009b. "Effect of prostaglandin F2alpha on subclinical endometritis and fertility in dairy cows." J. Dairy Sci. 92: 4906–13. https://doi.org/10.3168/jds.2008-1984
Gilbert, R.O., S.T. Shin, C.L. Guard, H.N. Erb, and M. Frajblat. 2005. "Prevalence of endometritis and its effects on reproductive performance of dairy cows." Theriogenology 64: 1879–88. https://doi.org/10.1016/j.theriogenology.2005.04.022
Goshen T., and N.Y. Shpigel. 2006. "Evaluation of intrauterine antibiotic treatment of clinical metritis and retained fetal membranes in dairy cows." Theriogenology 66: 2210–18. https://doi.org/10.1016/j.theriogenology.2006.07.017
Hammon, D.S., I.M. Evjen, T.R. Dhiman, J.P. Goff, and J.L. Walters. 2006. "Neutrophil function and energy status in Holstein cows with uterine health disorders." Vet. Immunol. Immunopathol. 113: 21–9. https://doi.org/10.1016/j.vetimm.2006.03.022
Hussain, A.M. 1989. "Bovine uterine defense mechanism: a review." J. Vet. Med. B. 36: 641–51. https://doi.org/10.1111/j.1439-0450.1989.tb00657.x
Huzzey, J.M., D.M. Veira, D.M. Weary, and M.A. von Keyserlingk. 2007. "Prepartum behavior and dry matter intake identify dairy cows at risk for metritis." J. Dairy Sci. 90: 3220–33. https://doi.org/10.3168/jds.2006-807
Kasimanickam, R., T.F. Duffield, R.A. Foster, C.J. Gartley, K.E. Leslie, J.S. Walton, and W.H. Johnson. 2004. "Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows." Theriogenology 62: 9–23. https://doi.org/10.1016/j.theriogenology.2003.03.001
Kasimanickam, R., T.F. Duffield, R.A. Foster, C.J. Gartley, K.E. Leslie, J.S. Walton, and W.H. Johnson. 2005. "The effect of a single administration of cephapirin or cloprostenol on the reproductive performance of dairy cows with subclinical endometritis." Theriogenology 63: 818–30. https://doi.org/10.1016/j.theriogenology.2004.05.002
LeBlanc, S.J., T.F. Duffield, K.E. Leslie, K.G. Bateman, G.P. Keefe, J.S. Walton, and W.H. Johnson. 2002. "Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows." J. Dairy Sci. 85: 2223–36. https://doi.org/10.3168/jds.S0022-0302(02)74302-6
Lewis, G.S. 2004. "Steroidal regulation of uterine immune defenses." Anim. Reprod. Sci. 82-83: 281–94. https://doi.org/10.1016/j.anireprosci.2004.04.026
McDougall, S. 2001. "Effect of intrauterine antibiotic treatment on reproductive performance of dairy cows following periparturient disease." N. Z. Vet. J. 49: 150–8. https://doi.org/10.1080/00480169.2001.36223
McDougall, S., R. Macaulay, and C. Compton. 2007. "Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle." Anim. Reprod. Sci. 99: 9–23. https://doi.org/10.1016/j.anireprosci.2006.03.017
Risco, C.A., and J. Hernandez. 2003. "Comparison of ceftiofur hydrochloride and estradiol cypionate for metritis prevention and reproductive performance in dairy cows affected with retained fetal membranes." Theriogenology 60: 47–58. https://doi.org/10.1016/S0093-691X(02)01299-2
Sheldon, I.M., and H. Dobson. 2004. "Postpartum uterine health in cattle." Anim. Reprod. Sci. 82-83: 295–306. https://doi.org/10.1016/j.anireprosci.2004.04.006
Sheldon, I.M., G.S. Lewis, S. LeBlanc, and R.O. Gilbert. 2006. "Defining postpartum uterine disease in cattle." Theriogenology 65: 1516–30. https://doi.org/10.1016/j.theriogenology.2005.08.021
Thurmond M.C., C.M. Jameson, and J.P. Picanso. 1993. "Effect of intrauterine antimicrobial treatment in reducing calving-to-conception interval in cows with endometritis." J. Am. Vet. Med. Assoc. 203: 1576–78. https://doi.org/10.2460/javma.1993.203.11.1576
Williams, E.J., D. P. Fischer, D.U. Pfeiffer, G.C. England, D.E. Noakes, H. Dobson, and I.M. Sheldon. 2005. "Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle." Theriogenology 63: 102–17. https://doi.org/10.1016/j.theriogenology.2004.03.017