What Is Malignant Catarrhal Fever (MCF)?
Malignant catarrhal fever (MCF) is a viral infection of a wide variety of ruminant species and, in rare cases, swine. The viruses responsible for this infection are known as gammaherpesviruses and circulate subclinically in reservoir species, most notably wildebeest (Connochaetes spp.) and sheep (Ovis aries). Infections of non-reservoir species are often fatal and have been cause for worldwide economic concern. Of the sheep-associated (ovine) and wildebeest-associated (alcelaphine) strains, the sheep-associated is the less studied, even though it is more common in the United States. There are also several strains that have been linked to domestic goats (Capra hircus, caprine).
There is limited data on MCF prevalence in the United States, especially within the state of Florida. Most studies have described outbreaks in farmed American bison (Bison bison), although none of those studies have detected MCF in Florida herds. White-tailed deer (Odocoileus virginianus), the most farmed cervid in Florida, have been found to have contracted MCF viruses in Missouri, North Dakota, Indiana, Ohio, Texas, and New Jersey. It has been detected in wild animals as well, such as free-ranging mule deer (Odocoileus hemionus) in Colorado. There is limited surveillance of the pathogen in both reservoir and incidental host populations, suggesting it is likely more widespread than currently documented.
What Are Common Clinical Signs of MCF?
Credit: Zhu et al. (2018); http://creativecommons.org/licenses/by/4.0/
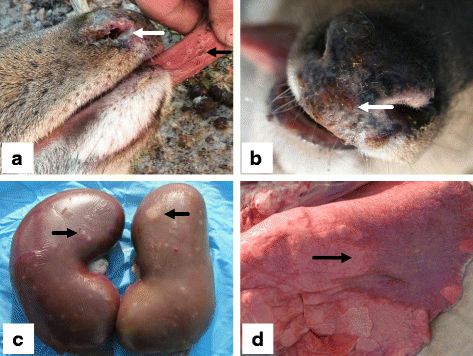
Infection of reservoir hosts is generally subclinical, and animals remain infected for their entire lifespan. Such covert infections may also occur in non-reservoir species. MCF is a lymphoproliferative disease, causing uncontrolled lymphocyte production. The dysregulation of this process and subsequent, inappropriate prevalence of immune cells can disrupt host responses to other infections. Symptoms of disease vary according to incidental host species but include many nonspecific clinical indicators like fever and general wasting. Infected cattle (Bos taurus and Bos indicus) may have discharge emanating from the eyes and nose, and both of their eyes may appear clouded (bilateral corneal opacity). Bison often die too quickly for these more indicative clinical signs to arise. Certain cervid species, such as Père David's deer (Elaphurus davidianus), white-tailed deer (Odocoileus virginianus), axis deer (Axis axis), and sika deer (Cervus nippon), are particularly susceptible to this virus. Those cervid species that are less susceptible can survive for up to three weeks after the onset of initial symptoms. Fallow deer (Dama dama) have been noted as being particularly resilient.
The oculonasal discharge commonly reported in cattle is less common in deer, while hemorrhagic diarrhea and bloody urine are more common. Yet another strain of MCF, a goat-associated (caprine) virus (of which there are three), is reported to cause alopecia (hair loss) and hyperkeratosis, a condition characterized by the thickening of the outer layer of the skin in captive white-tailed (Odocoileus virginianus) and sika (Cervus nippon) deer. Upon necropsy, most incidental hosts present with inflammation of the mucous membranes and corresponding necrosis and ulceration. Several organ systems have been found affected by gross lesions, and histopathological examination of these lesions often reveals hemorrhage and infiltration of lymphoid cells. The lymph nodes may be swollen and apparent even in early stages of infection.
How Do Non-Reservoir Species Contract MCF?
There is little evidence that MCF viruses are transmissible between members of non-reservoir species; thus, they are most likely to be contracted through interaction with a reservoir species or associated biomaterials like feces. Sheep and wildebeest shed virus predominantly from the respiratory tract, either as secretions or aerosols. Incidental hosts inhale or ingest shed viral particles from these products. While MCF viruses may be transmitted on surfaces, they are viable only for short periods of time. Virus survival has been found to increase with humidity. Alcelaphine MCF is endemic in several areas across Africa that have seasonal peaks in case load, whereas case numbers in the United States increase sporadically as mediated by specific incidents of reservoir and incidental host interaction.
How Is MCF Infection Prevented?
There is no available vaccine for MCF. Studies of American bison (Bison bison) have found that MCF outbreak severity was significantly correlated with distance from sheep feedlots, with increased distance corresponding to lower fatality. Some herds contracted MCF from as far as 5 km from sheep feedlots. Maintaining distance between potential reservoirs and susceptible incidental hosts is the most effective strategy for management and prevention of MCF. Sheep and goat farms are widespread in the state of Florida, and these species may be reared in proximity to cattle or susceptible non-native and native cervids. If this is the case, precautions must be taken to ensure susceptible animals do not come into contact with reservoir animals or contaminated materials from their respective farms. As with other diseases, livestock markets and auctions pose a significant risk for spillover, as they bring a variety of animals into close contact at high densities from different regions. Enhancing the overall health and welfare of susceptible animals can mitigate risk and involves managing established stressors, such as overcrowding and poor nutrition.
How Is MCF Infection Treated?
Prevention is the best way to ensure the survival of susceptible animals. There is no vaccine combating MCF virus infections, and there is currently no well-supported treatment for MCF. Nonetheless, the treatment of viral diseases typically involves providing relief for clinical signs and offering supportive care. Preventing secondary bacterial infections with antibiotics and good hygiene is also important for the survival of infected animals. Isolation and containment measures for infected individuals are essential for preventing the spread of the virus. Subclinical infections persist throughout the entirety of a reservoir host’s life. Lambs and kids in infected sheep and goat herds contract these viruses within the first few months of life, generally after the 2-month mark. Because of the near ubiquity of infection in affected herds, the best method of prevention is total separation of reservoir and susceptible species. Diagnostic testing (by conventional PCR) is available in-state for MCF viruses and may be accessed via veterinary services. Seeing as transmission between susceptible animals is unlikely, it is not recommended that sick animals be culled. It is still recommended that sick animals be separated from susceptible, uninfected animals. If populations of susceptible and reservoir host species are to be kept together or in proximity to each other, preliminary testing of the reservoir population may prevent die-off in the susceptible population. Palliative care and good husbandry may coax an animal through the infection, but prognosis is grave. Case fatalities are high, and treatment is typically unrewarding.
Summary
- MCF is a lymphoproliferative disease caused by a group of viruses that circulate subclinically in herds of wildebeest, sheep, and goats.
- When incidental hosts such as cattle, bison, or deer contract one of these viruses, MCF is often fatal.
- MCFVs are not known to be transmissible between incidental hosts; they can only be transmitted by contact between reservoirs and incidental hosts.
- The best way to prevent infection of susceptible incidental hosts is to keep them separate from potential reservoirs.
- There is currently no specific treatment for MCF.
References
Aiello, S. E., and M. A. Moses. 2016. “Malignant Catarrhal Fever.” In The Merck Veterinary Manual. 11th ed. 758–760. Merck and Co.
Berezowski, J. A., G. D. Appleyard, T. B. Crawford, J. Haigh, H. Li, D. M. Middleton, B. P. O’Connor, K. West, and M. Woodbury. 2005. “An Outbreak of Sheep-Associated Malignant Catarrhal Fever in Bison (Bison bison) After Exposure to Sheep at a Public Auction Sale.” Journal of Veterinary Diagnostic Investigation 17 (1): 55–58. https://doi.org/10.1177/104063870501700110
Brown, C. C., and L. L. Bloss. 1992. “An Epizootic of Malignant Catarrhal Fever in a Large Captive Herd of White-Tailed Deer (Odocoileus virginianus).” Journal of Wildlife Diseases 28 (2): 301–305. https://doi.org/10.7589/0090-3558-28.2.301
Kleiboeker, S. B., M. A. Miller, S. K. Schommer, J. A. Ramos-Vara, M. Boucher, and S. E. Turnquist. 2002. “Detection and Multigenic Characterization of a Herpesvirus Associated with Malignant Catarrhal Fever in White-Tailed Deer (Odocoileus virginianus) from Missouri.” Journal of Clinical Microbiology 40 (4): 1311–1318. https://doi.org/10.1128/jcm.40.4.1311-1318.2002
Lankester, F., A. Lugelo, R. Kazwala, J. Keyyu, S. Cleaveland, and J. Yoder. 2015. “The Economic Impact of Malignant Catarrhal Fever on Pastoralist Livelihoods.” PLoS ONE 10 (1): e0116059. https://doi.org/10.1371/journal.pone.0116059
Li, H., C. W. Cunha, B. Abbitt, T. W. deMaar, S. D. Lenz, J. R. Hayes, and N. S. Taus. 2013. “Goats Are a Potential Reservoir for the Herpesvirus (MCF-WTD), Causing Malignant Catarrhal Fever in Deer.” Journal of Zoo and Wildlife Medicine 44 (2): 484–486. https://doi.org/10.1638/2012-0087R.1
Li, H., C. W. Cunha, N. S. Taus, and D. P. Knowles. 2014. “Malignant Catarrhal Fever: Inching Toward Understanding.” Annual Review of Animal Biosciences 2 (2): 209–233. https://doi.org/10.1146/annurev-animal-022513-114156
Li, H., G. Karney, D. O’Toole, and T. B. Crawford. 2008. “Long Distance Spread of Malignant Catarrhal Fever Virus from Feedlot Lambs to Ranch Bison.” The Canadian Veterinary Journal 49 (2): 183–185.
Reid, H. W., and D. Buxton. 1984. “Malignant Catarrhal Fever of Deer.” Proceedings of the Royal Society of Edinburgh, Section B: Biological Sciences 82 (4): 261–273. https://doi.org/10.1017/S026972700000378X
Schultheiss, P. C., J. K. Collins, L. E. Austgen, and J. C. DeMartini. 1998. “Malignant Catarrhal Fever in Bison, Acute and Chronic Cases.” Journal of Veterinary Diagnostic Investigation 10 (3): 255–262. https://doi.org/10.1177/104063879801000305
Schultheiss, P. C., H. Van Campen, T. R. Spraker, C. Bishop, L. Wolfe, and B. Podell. 2007. “Malignant Catarrhal Fever Associated with Ovine Herpesvirus-2 in Free-Ranging Mule Deer in Colorado.” Journal of Wildlife Diseases 43 (3): 533–537. https://doi.org/10.7589/0090-3558-43.3.533
Spickler, A. R. 2019. “Malignant Catarrhal Fever.” http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php
USDA National Agricultural Statistics Service. n.d. 2022 Census of Agriculture. www.nass.usda.gov/AgCensus
Zhu, H., Q. Huang, X. Hu, W. Chu, J. Zhang, L. Jiang, X. Yu, X. Zhang, and S. Cheng. 2018. “Caprine Herpesvirus 2-Associated Malignant Catarrhal Fever of Captive Sika Deer (Cervus nippon) in an Intensive Management System.” BMC Veterinary Research 14 (1): 38. https://doi.org/10.1186/s12917-018-1365-8