Atlantic Croaker
General Description
The Atlantic croaker, Micropogonias undulatus (Figure 1), is a member of the family Sciaenidae, the drum family. This medium-sized fish is slightly elongate, moderately compressed, and silvery in color with a pinkish cast; the back and upper sides are grayish with black spots forming irregular, oblique lines above the lateral line. The dorsal fin has small black dots and a black edge; other fins are pale to yellowish. The chin has 3 to 5 pairs of barbels along the inner edge of the lower jaw.
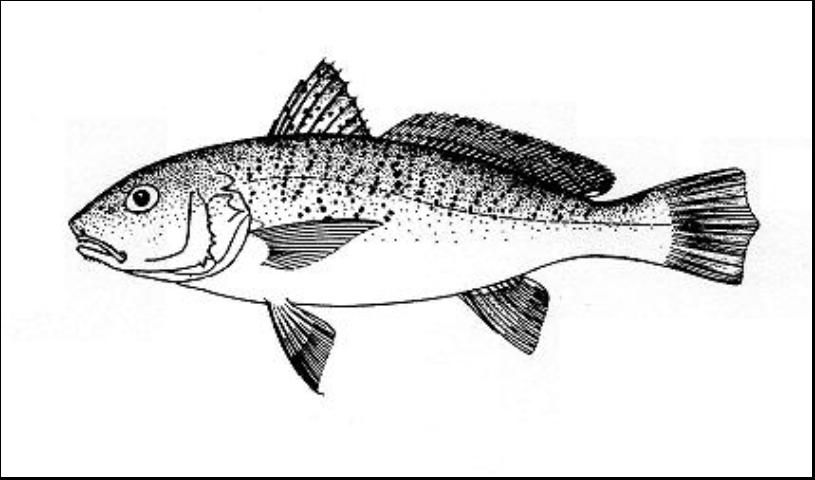
Credit: Food and Agriculture Organization of the United Nations (1978)
Geographic Distribution and Habitat
The Atlantic croaker is known to occur in the northern and eastern parts of the Gulf of Mexico, along the Atlantic coast of the United States from south Florida to Massachusetts, in the Greater Antilles, and along the South American Atlantic coast from Surinam to Argentina. Its US fishing grounds extend from the Rio Grande to Tampa Bay in the Gulf of Mexico and from northern Florida to Cape Hatteras on the Atlantic coast. In Florida, Atlantic croaker are seldom found south of Tampa Bay or the Indian River Lagoon on the Atlantic coast (Lankford and Targett 1994). Croaker are found over mud and sandy mud bottoms in coastal waters to about 1,000 meter depth and in estuaries where the nursery and feeding grounds are located.
Natural History
Atlantic croaker are medium-sized members of the drum family, usually less than 14 inches long when caught and achieve a maximum age of about 8 years (Barbieri et al. 1994). They mature at the end of the first or second year when they are 6 to 10 inches long, spawning over the nearshore continental shelf during the late-fall and winter (White and Chittenden 1977). Post-larval and juvenile Atlantic croaker occupy estuarine nursery areas where they feed on benthic plankton and invertebrates, such as grass shrimp and worms (Mercer 1987). Croaker are preyed upon by a variety of fish, including striped bass, southern flounder, spotted seatrout, red drum, sharks, and others carnivorous fishes.
Atlantic croaker are euryhaline, which means they occur in a wide range of salinities from 0 ppt to full seawater at 35 ppt and into hypersaline waters of more than 35 ppt (ppt = parts per thousand). Croakers are also eurythermal, and can be found in waters with a bottom temperature range of 9°C–32°C (48°F–90°F); they are most abundant in waters over 24°C (75°F) (Miglarese et al. 1982).
Culture Methods
Broodstock
Aquaculture methods for Atlantic croaker are only partially known. The reproductive biology and spawning of wild-caught fish is well-documented (Avault et al.1969; Wallace 1940; Fruge and Truesdale 1978). Atlantic croaker mature at a small size and early age. The estimated mean total length of 50% (L50) of the males at first maturity is 182 mm (7.2 inches) and 173 mm (6.8 inches) for females. More than 85% of both males and females are sexually mature by the end of their first year, and all are mature by the end of their second (Barbieri et al. 1994).
Croakers spawn along the mid-Atlantic coast over a protracted period, typically from July to December, but spawning may occur throughout the year in Florida waters. Individual fish apparently spawn over a shorter period, lasting three to four months. Croakers have been successfully induced to spawn using human chorionic gonadotropin (125 I.U.) administered three times per week for three weeks. An apparent period of latency (6–7 days) occurs after the first injection resulting in successful ovulation (Middaugh and Yoakum 1974). Fecundity estimates of up to 180,000 eggs for a 39 cm (15.3 inch) female have been reported.
Hatchery
Larvae have been reared in cylindrical, fiberglass tanks containing 200–300 L of seawater. Water quality is maintained with the use of bio-filters or by traditional flow-through methods. These fish are initially raised at environmental parameters close to that of the spawning tank and may be gradually acclimated to ambient conditions if necessary. Each larval tank should be gently aerated.
Newly hatched Artemia nauplii (@450 microns) are too large for croaker larvae to ingest at first feeding. Beginning at day three, post-hatch, rotifers (Brachionus sp.) are a common food source for marine fish larvae due to their smaller size (90–250 microns) although they must be cultured as a live feed. Houde and Ramsey (1971) successfully reared croaker larvae under static conditions by maintaining them for approximately one week in a dense culture of Chlorella phytoplankton followed by a slow water exchange rate with copepod nauplii and other wild zooplankters introduced at that time. Further research feeding copepod nauplii to early larval stages is warranted. However, recommended hatchery production and feeding regimes for croaker have been successful following standard practices for red drum (Sciaenops ocellatus) (Chamberlain et al. 1990). Best results are obtained when larvae are reared in a static culture of Isochrysis galbana (phytoplankton) or similar algae with high levels of docosahexanoic acid (DHA). Algae should be added to culture tanks by 3 days after hatching when larvae are ready to feed. Rotifers should be fed on days 3–12 post-hatch. At first feeding, rotifers should be maintained in the rearing tanks at a concentration of 5/ml. Depending on the density of larvae, rotifers may need to be added to the tanks 2–3 times per day to maintain this concentration. Microparticulate diets (250 micron particle size) can be added starting on day 6 at a rate of 0.001g/L/day. Dry feed should be added to the tanks by hand several times a day or with the use of a continuous feeder. A small amount of Artemia nauplii (0.25–0.5/ml) should be added to the rearing tanks 10–12 days after hatching along with the rotifers to help wean larvae off rotifers. Artemia nauplii alone should be added to the tanks at a concentration of 0.5-1/ml on days 13 through metamorphosis (approximately day 25). If the fish are clearing the tank of Artemia in just a few hours, additional nauplii should be added to the tank in the afternoon. On day 13, increase the particle size for dry feed to 400 microns. Dry feed should be added to the tank by hand several times per day or with the use of a time-released or continuous feeder at a rate of 0.01g/L/day. Each day, increase the amount of dry feed by 0.001g/L or as needed. Observe the tank closely, removing any dry food that accumulates in the tank to prevent spikes in ammonia and nitrite levels. At metamorphosis, larvae are moved from the larval rearing tanks to the growout system (Chamberlain et al. 1990).
Nursery
Juvenile croakers respond well to high protein (45%) formulated diets and exhibit rapid growth and survival in cage systems, > 400% weight gain over a 7 week period at 28°C (82°F) and 28 ppt (Davis and Arnold 1997; Jones and Strawn 1983). In the wild, juvenile croakers grow rapidly, reaching 6 to 8 inches in the first year, however their performance in culture depends on a variety of parameters such as culture system (tank vs. pond), environmental conditions (temperature/salinity regimes), and diet (type of feed and frequency of feeding).
Disease
Although there is no documentation of the susceptibility of croaker to various disease-causing organisms, several related species, in particular red drum, are known to suffer heavy mortalities due to pathogens during larval and juvenile stages. However, disease outbreaks can be prevented by avoiding conditions that may predispose the fish to pathogens due to stress. Common environmental stressors include poor water quality, excessive stocking density, and inadequate nutrition. The most common pathogen impacting red drum culture is the parasitic dinoflagellate Amyloodinium ocellatus. This organism attacks the gills of red drum resulting in reduced oxygen uptake of the fish. Salinity appears to influence the occurrence of Amyloodinium ocellatus infestations. Fish are generally less susceptible at salinities below 6 ppt (Gatlin 2000).
Growout
Information about growout of juvenile croaker to harvest size for bait is lacking. On average, croaker sold for bait range from 5 to 10 inches (127–254 mm total length; TL). Jones and Strawn (1983) grew juvenile croakers (collected from the wild using an otter trawl) in floating cylindrical cages (0.6 m diameter x 0.6 m depth = 0.18 m3) in heated effluent from a Texas power plant. Twenty-five fish were stocked per cage and fed formulated feeds (45% protein, 5% lipid). The water temperature remained steady at 30°C (86°F), which is higher than required for maximum growth and may have resulted in a slight decline in growth, while salinity ranged from 0 to 16 ppt. During 90-day trials, croaker juveniles grew 0.37 to 0.54 mm/day and 0.23 to 0.41 g/day; production ranged from 3.1 to 5.4 kg/m3 (6.8–11.9 lb/ m3). Survival ranged from 80 to 100%. Florida baitfish retailers sell croaker at a size range from 5 to 10 inches total length (Adams et al. 1998); based upon growth rates reported by Jones and Strawn, production time from a 1-inch fingerling to a 5-inch harvest size would take approximately 8.5 months. This study clearly demonstrates that Atlantic croaker are highly euryhaline (they adapt to a wide range of salinity), however their ability to quickly acclimate from freshwater to seawater has not been determined.
Experiments in Texas estimated that feed conversion efficiencies for croaker maintained on the 45% protein diet with 8% lipid (primarily from fish meal and soybean meal) were significantly higher than those maintained on the 45% protein diet containing 16% lipid. Based on the observed results, the Atlantic croaker appears to grow best on high protein diets with moderate lipid levels (Chamberlin et al. 1990).
Marketing
In Florida, the majority of Atlantic croaker are landed by recreational anglers (89% by weight), and commercial landings have declined due to the low value of this species as a food fish ($0.46/pound). Therefore, farming of Atlantic croaker for the food fish market is not likely economically feasible. However, croaker is a species of choice as a baitfish for grouper and spotted seatrout. A summary assessment of the market value for croaker in Florida reported a wholesale price (per fish) of $0.19, with a retail price per dozen of $8.90, although the size of the fish was not reported (Adams et al. 1998). Further market research on cultured croaker as a potential baitfish is justified.
Summary of Aquaculture Potential in Florida
Although there is not a significant body of information regarding the culture of Atlantic croaker, it is likely amenable to aquaculture as are other sciaenid fish such as red drum and spotted seatrout. Since it is a hardy fish, easily maintained in captivity, and readily acclimated to a wide range of environmental conditions, it deserves strong consideration as a candidate species for baitfish aquaculture. A workshop dedicated to candidate species for baitfish aquaculture in Florida (sponsored by University of Florida Sea Grant) ranked the Atlantic croaker as one of the priority species that "demonstrated the greatest potential for successful development as a live bait for the recreational angling community" (Oesterling et al. 2004).
References
Adams, C.M., A.M. Lazur, P. Zajicek, and D. Zimet. 1998. An assessment of the market for live marine baitfish in Florida. Bureau of Seafood and Aquaculture, Florida Department of Agriculture and Consumer Services. 32 pp.
Avault, J.W., Jr., C.L. Birdsong, and W.O. Perry. 1969. Growth, survival, food habits and sexual development of croaker, Micropogonias undulatus, in brackish water ponds. Proceedings of the Southeastern Association Game and Fish Commission 23: 251–255.
Barbieri, L.R. M.E. Ctittenden, Jr., and S.l.K. Lowerre-Barbieri. 1994. Maturity, spawning, and ovarian cycle of Atlantic croaker, Micropogonias undulatus, in the Chesapeake Bay and adjacent coastal waters. Fisheries Bulletin 92(4): 671–685.
Chamberlain, G.W., R.J. Miget, and M.G. Haby. 1990. Red Drum Aquaculture. Texas A & M University Sea Grant College Program, TAMU-SG-90-603. 236 pp.
Davis, D.A. and C.R. Arnold, Jr. 1997. Response of Atlantic croaker fingerlings to practical diet formulations with varying protein and energy content. Journal of the World Aquaculture Society 28(3): 241–248.
Fruge, D.J. and F.M. Truesdale. 1978. Comparative larval development of Micropogonias undulatus and Leiostomus xanthurus (Pisces: Sciaenidae) from the northern Gulf of Mexico. Copeia 1978(4): 643–648.
Gatlin, D.M., III. 2000. Red drum culture. Pages 736–742 In: R.R. Stickney (ed.) Encyclopedia of Aquaculture. New York, NY: John Wiley & Sons.
Houde, E.D. and A.J. Ramsey. 1971. A culture system for marine fish larvae. Progressive Fish Culturist 33(3): 156–157.
Jones, F.V. and K. Strawn. 1983. Growth and food utilization of caged Atlantic croaker and striped mullet reared on various lipid diets in a heated water system. Journal of the World Mariculture Society 14: 590–594.
Lankford, T.E. and T.E. Targett. 1994. Suitability of estuarine nursery zones for juvenile weakfish (Cynoscion regalis) effects of temperature and salinity on feeding, growth, and survival. Marine Biology 119: 611–620.
Mercer, L.P. 1987. Fishery management plan for Atlantic croaker (Micropogonias undulatus). Fisheries Management Report No. 10 of the Atlantic States Marine Fisheries Commission. 90 pp.
Middaugh, D.P., and R.L. Yoakum. 1974. The use of chorionic gonadotropin to induce laboratory spawning of the Atlantic croaker, Micropogonias undulatus, with notes on subsequent embryonic development Chesapeake Science 15(2): 110–114.
Miglarese, J.V., C.W. McMillian, and M.H. Sealy, Jr. 1982. Seasonal abundance of Atlantic croaker (Micropogonias undulatus) in relation to bottom salinity and temperature in South Carolina estuaries. Estuaries 5: 216–223.
Oesterling, M.J., C.M. Adams, and A.M. Lazur. 2004. Marine baitfish culture: workshop report on candidate species & considerations for commercial culture in the southeast U.S. Virginia Sea Grant Program, Marine Resource Advisory No. 77. 27 pp.
Wallace, D.H. 1940. Sexual development of the croaker, Micropogonias undulatus, and distribution of the early stages in Chesapeake Bay. Transactions of the American Fisheries Society 70: 475–482.
White, M.L. and M.E. Chittenden, Jr. 1977. Age determination, reproduction and population dynamics of the Atlantic croaker, Micropogonias undulatus. Fisheries Bulletin 75:109–123.