Once the HACCP foundation (pre-steps and prerequisite programs) is in place, the HACCP team must conduct a hazard analysis. Hazard Analysis (Principle 1) must be thorough and complete, as it provides the scientific basis for the HACCP plan. During this step, certain elements of the preliminary steps (e.g., product description, intended use, flow diagram) are reassessed with respect to potential hazards.
Sufficient time and energy must be spent on this important HACCP principle. Performing an effective and efficient hazard analysis is one of the most difficult steps in the HACCP Program, and generally takes the majority of the total time in the process. During hazard analysis, there is often a tendency to jump ahead to identify Critical Control Points (CCPs). This is inappropriate, as it may jeopardize the scientific validity of the plan. Moreover, an important training opportunity for the HACCP team may be lost by doing this.
I. HACCP Definition of a Food Hazard
According to the widely accepted HACCP definition, a hazard is a significant biological, chemical, or physical hazard that can cause harm and that is reasonably likely to occur if not controlled. With regard to HACCP Hazard Analysis, the working elements of this definition are "significant" and "likely to occur." While these may be separate components, they are usually interrelated. In addition to identifying and evaluating hazards, an adequate hazard analysis also involves identifying preventative and control measures.
A. Biological Hazards
This category of food hazards poses the greatest risk for illness or injury. Biological hazards include bacteria, fungi, viruses, and parasites. These hazards vary widely in their prevalence, mode of action, infectious dose, growth and survival characteristics, and resistance to heating, chemical agents, and other food processes or treatments. The following categories should be used and considered when performing a HACCP hazard analysis.
1. Pathogenicity of Biological Hazards
In order for a pathogen to cause illness, a host must be susceptible. Age, size, weight, consumption and vulnerability all play a role. The pathogenicity of the biological agent itself is also relevant. Foodborne pathogens fall into two general categories: food intoxications and foodborne infections.
In food intoxications, the levels of a biological agent in a food are high enough to produce a toxin which, upon ingestion, causes illness. Cases of food intoxication usually involve temperature abuse of the product, causing bacterial growth that leads to harmful levels of toxins. Foodborne illness caused by toxins usually results in the rapid onset of nausea and vomiting.
In foodborne infections, the human host ingests a sufficient quantity of a living biological agent to become sick (this is referred to as the "infectious dose"). The symptoms and onset time are highly variable, and depend upon the characteristics of the agent and the host. The two basic types of foodborne infection are as follows:
- Invasive infections: The biological agent penetrates or invades cells and tissues in the host to cause illness.
- Toxico-infections: The biological agent takes up residence in the intestinal walls, producing a toxin that causes illness.
2. Categories of Biological Hazards
- Bacteria. Bacteria represent the largest and most diverse category of potential food hazards. In addition to being categorized as either food intoxications or foodborne infections, bacterial hazards are further categorized according to their growth and survival characteristics.
With regard to growth temperature, bacteria are generally categorized as follows:
- Thermophiles: Optimum growth at relatively high temperatures (45°C or above)
- Mesophiles: Optimum growth range of 20°C to 45°C
- Psychrotrophs: Mesophiles that will grow under refrigeration conditions
The majority of foodborne pathogens are mesophiles. Some foodborne pathogens are psychrotrophs and capable of growing slowly under refrigeration.
Spore-forming bacteria are capable of existing in a protective extracellular capsule and are, thus, highly resistant to heat treatment and other food processes. Vegetative bacteria (which do not produce the extracellular spore) are generally less resistant to heating and other processes. If subjected to sublethal heat treatment, spore-forming bacteria may germinate (as vegetative cells) and grow, depending upon conditions. Bacteria also vary in their resistance to food acidity or pH, oxygen levels, low water activity (dryness), and other chemicals used in food processing and handling.
- Fungi. The fungi associated with foods are generally yeasts and molds. Of greatest concern for food safety are the mycotoxins (e.g., aflatoxin, fusarin, patulin), which are produced by molds and may be associated with chronic illnesses, such as cancer. These toxins are addressed as chemical hazards in the HACCP hazard analysis.
- Viruses. Viruses, which are considerably smaller than bacteria, are primarily composed of protein and nucleic acids. Illnesses caused by viruses present in food are classed as foodborne infections. Viruses are host-specific (human viruses affect human hosts), and their presence in food is associated with human waste contamination. The majority of foodborne illnesses related to viruses can be traced to contaminated water coming into contact with food, improper handwashing, or improper personal hygiene. Foodborne viruses are generally not heat-resistant. Further, they do not grow on culture media, do not multiply in foods, and do not survive for long periods without a host. The viruses which cause the most foodborne illness include hepatitis A and noroviruses.
- Parasites. Like viruses, parasites generally do not multiply in food products. They also require a host. The illnesses caused by parasites can be classed as foodborne infections. Individual susceptibility varies depending on the host.
The most common parasites associated with foods are the microscopic protozoan parasites (Cryptosporidium parvum, Cyclospora cayetanensis, Giardia lamblia, Trichinella spiralis, and Toxoplasma gondii). Cryptosporidium, Cyclospora, and Giardia are usually waterborne, but have been associated with fresh produce in recent years. Trichinella and Toxoplasma have been associated with undercooked pork and other undercooked meats. Other parasites (e.g., nematodes, tapeworms, flukes) have been associated with seafood and seafood products to varying degrees.
B. Chemical Hazards
A wide variety of chemical residues can occur in foods as a result of chemical usage in food production and processing. Most of these chemicals are regulated substances under federal regulatory agencies. Accidental or environmental chemical residues can also occur in foods under certain conditions. With regard to hazard analysis, chemicals of concern include: naturally occurring substances, unavoidable poisonous or deleterious substances, toxins of microbial origin (e.g., histamine or scombrotoxin in fish, mycotoxins), allergens, food additives, antibiotics and hormones, pesticides, cleaning and sanitizing agents, industrial chemicals and pollutants, and others deemed appropriate.
C. Physical Hazards
Many foreign material residues in food (e.g., hairs, filth) are regulated under FDA Defect Action Levels (see https://www.fda.gov). Other physical hazards which may be of sufficient size to cause harm (e.g., glass, metal or wood fragments, packaging materials, hypodermic needles, bullets and related materials, stones) must be addressed in the HACCP hazard analysis.
II. Scope of Hazard Analysis
Unlike HACCP principles 2 through 6, which are usually limited to operations within the facility's control, hazard analysis has a much broader scope and must include factors both within and outside of the facility. The following is a listing of what should be included (at a minimum) in the hazard analysis:
- Ingredients and raw materials: source, composition, handling, transportation, and storage.
- Activities conducted in the process and handling system: steps identified on the flow diagram.
- Equipment used in manufacture and processing: specific parameters important to controlling, reducing, or preventing hazards.
- Equipment and facility sanitation.
- Food product distribution: transportation, delivery, wholesale/retail practices, and intended use.
Documentation of the hazard analysis should include a written summary providing relevant scientific references. In addition, the information should be summarized in a narrative statement and listed on appropriate forms.
III. Elements of Hazard Analysis
The hazard analysis must be specific and pertinent to the food processing and handling system in question. Generic, one-size-fits-all hazard analyses are not appropriate. While there are many sources of data and generic information available, procedures must be tailored to your specific situation, and should involve thorough investigation by the HACCP team. There are many recommendations as to how to conduct a hazard analysis. Most of these involve some version of the following two components:
- Hazard Identification: Identifying and developing a list of potential hazards.
- Hazard Evaluation: Assessing the relative risks, severity, significance, and likelihood of occurrence of the potential hazard in the specific food processing and handling system.
In conducting a hazard analysis, the team should consult as many scientific sources as possible (see reference list for examples), and should evaluate historical data, past experiences, and any scientific experiments conducted by the company, or a third party on behalf of the company.
A. Hazard Identification
During the hazard identification stage, the HACCP team should critically examine all elements within the scope of hazard analysis by listing all potential biological, chemical, and physical hazards associated with each material, ingredient, activity or step used in the food processing and handling system. The hazards list should be identified (listed) on an appropriate form. If possible, it is recommended that hazards on the list be grouped according to their general characteristics.
B. Hazard Evaluation
This stage involves analysis of the significance and likelihood of occurrence of the food hazards listed in the hazards list. While this is often not a thorough academic scientific risk assessment, it does possess elements of risk assessment.
During hazard evaluation, the severity of hazards should be evaluated in light of the specific conditions and situations anticipated for the specific food products manufactured, and within the scope of the HACCP plan. The hazard evaluation should include the following:
- Effectiveness of prerequisite programs.
- Frequency of association of the hazard with the type of food or ingredients.
- Frequency of occurrence within the facility.
- Food product composition and other intrinsic factors (e.g., pH, water activity) which may enhance or inhibit growth.
- Extrinsic factors (e.g., oxygen level, temperature considerations).
- Processing considerations.
- Storage and transportation conditions.
- Likely preparation steps by consumers.
- Probable consumption information and risk factors (e.g., expected level, target consumer).
1. Codex Alimentarius Risk Assessment Grid
Under Codex Alimentarius HACCP recommendations, a risk assessment grid is used (Table 1). While an effective tool in hazard analysis and team training, the grid system may create some confusion in application. In the grid system, the relative risk of a hazard is assessed by comparing severity (low, medium, high) and likelihood of occurrence (remote, low, medium, high). It is clear that a hazard with a rating of H/H has a high severity, is highly likely to occur, and should be addressed, and that one rated L/L may not need to be addressed. Meanwhile, a rating of H/R would describe a hazard that is very severe, but is only remotely likely to occur in the specific food system; thus, it is not "significant" and need not be addressed in the HACCP plan. However, there is some confusion with regard to some of the midrange ratings.
2. Assessing Hazards Using a Numerical Scale
To alleviate some of the confusion in using the grid system, some HACCP trainers and auditors have recommended using a numerical scale to characterize food hazards. For example, in the system recommended by Nolan, severity and frequency are each ranked using a 10-point ranking scale. The HACCP team sets the numerical combined score value that they consider to be significant. This system, though arbitrary, may have merit, especially in training the HACCP team in the evaluation phase of hazard analysis.
3. Simplified Hazard Analysis
In current application of the HACCP system, a more simplified approach is often used. In these simplified approaches, the Codex Risk Assessment Grid and other related assessment tools are not used. This may be appropriate, as long as the HACCP team is thorough in their evaluation and team training opportunities are not lost.
IV. Forms Used in Hazard Analysis
Several types of forms and worksheets may be used in the hazard analysis. These range from fairly simple to more complex. Some of these forms are described below.
A. Hazard Identification Forms
Fairly simplified hazard identification forms are presented in Figure 1. Materials/ingredients or processing steps are listed in the first column, along with potential hazards introduced for each material and ingredient or at each processing step. Generally, separate forms are used for materials/ingredients and for processing steps. Hazards should be listed in specific detail. More generic terminology (e.g., pathogens, chemicals) should be avoided. A listing of where the hazard is controlled is given in the second column. Hazards with similar characteristics (e.g., spore-formers, vegetative cells) may be grouped together on the form.
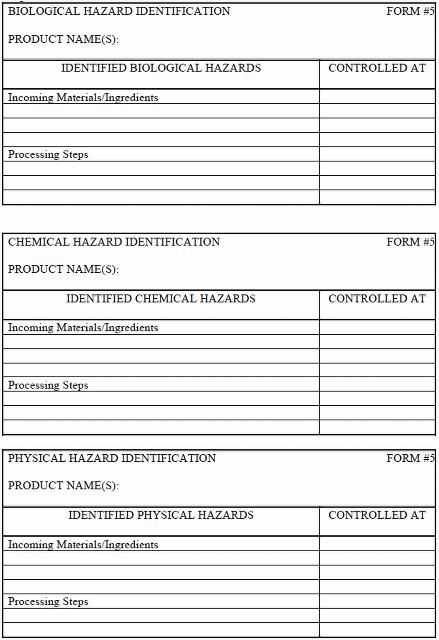
B. Hazard Analysis Worksheets
The most commonly used Hazard Analysis Worksheets are presented in Tables 2 and 3. These forms allow for more detail in the hazard evaluation than the form shown in Figure 1. While worksheets are similar, there are notable differences, especially in hazard evaluation. Keep in mind that each of them has advantages and disadvantages in application. It is up to your team to decide which form is appropriate for your specific HACCP program. If you are operating under a HACCP regulation, it is recommended that the forms used be compatible with those recommended by the responsible regulatory agency.
Column 1
It is recommended that separate forms be used for materials and ingredients and for processing steps. A thorough listing of all pertinent items should be listed on the Materials and Ingredients form. The form for processing steps should follow the flow diagram developed as part of the pre-step activities.
Column 2
As discussed above, when listing the food hazards, it is important to be specific and provide detailed information. If there are several potential hazards with similar characteristics, they can be grouped together with referenced footnotes to explain the criteria used for making this decision. For example, when listing biological hazards, terms like "vegetative pathogens," "spore-forming pathogens," "infectious pathogens," or "toxin-producing pathogens" may be used with a footnote listing the specific pathogens. Another option would be to identify the specific pathogens in the hazard analysis summary.
Column 3
It is in Columns 3 and 4 that the hazard evaluation phase of hazard analysis is documented. In the form shown in Table 2, the HACCP team is asked to assess the severity or significance of each hazard. This assessment may be derived from the Codex grid, or by using common sense and subjective terms. It is important that this assessment be justified by scientific or other information. This justification is listed in Column 4. In the form presented in Table 3, the hazard significance is assessed by asking the question, "Does the potential hazard need to be addressed in the HACCP plan?" However, this question can be a little misleading and confusing, since, technically speaking, all hazards are addressed in the HACCP plan (if only in the hazard analysis portion).
Column 4
Column 4 asks the team to "justify their decision" as to the significance of the hazard assessed in Column 3. This column is often improperly used and can be a source of confusion. Ideally, the information given should be the scientific or other justification for the decision, based on hazard significance and frequency of occurrence, as shown in Column 3. This justification is based upon the hazard analysis and provides documentation regarding the thought process for this analysis.
In traditional HACCP application, control measures should not be listed in Column 4, but more appropriately in Column 5. It has become commonplace, however, to list certain control measures (e.g., prerequisite programs, SOPs, metal detectors) in Column 4 to show that the hazard is not "reasonably likely to occur" because of these program(s). This leads to duplication, as these measures are listed again in Column 5. A better alternative may be to simply put "not likely to occur" in Column 4 and list the control measure appropriately in Column 5.
Column 5
The information in Column 5 of the hazard analysis provides a template for critical control point determination (HACCP principle 2). Thus, it is important that all preventative and control measures be appropriately listed in the appropriate row on the form.
Column 6
The forms in widespread use for evaluating hazards for each process step provide the opportunity for the HACCP team to indicate whether this step is a CCP in Column 6. This information, while helpful in HACCP audits and verification, may, unfortunately, lure the HACCP team into making a premature decision regarding CCPs. Therefore, this information should not be entered on the form until after completion of HACCP Principle 2.
Summary
The HACCP plan is built upon a thorough hazard analysis. Whichever procedures are followed, it is imperative that this process is based upon current, scientifically valid information. If the hazards identified and evaluated in the Hazard Analysis are incorrect or inappropriate to the food system, the HACCP plan is destined for failure. The Hazard Analysis requires constant review and update based upon evolving science and technology, as well as any modification in the food facility.
References
Codex Alimentarius. (2003). Hazard analysis and critical control point (HACCP) system and guidelines for its application. ANNEX to Recommended International Code of Practice/General Principles of Food Hygiene. CAC/RCP 1-1969, Rev 4. FAO/WHO Codex Alimentarius Commission.
Mead, P. S., Slutsker, L., Dietz, V., McCaig, L., Bresee, J. S., Shapiro, C., Griffin, P. M., & Tauxe, R. (1999). Food-related illness and death in the United States. Retrieved December 8, 2022, from https://doi.org/10.3201/eid0505.990502
National Advisory Committee on Microbiological Criteria for Foods (NACMCF). (1992). Hazard analysis and critical control point systems. International Journal of Food Microbiology, 16, 1-23.
Nolan, M. (2007). Personal communication. S.A.F.E. Food Consulting Services.
Scott, V. N., & Stevenson, K. E. (2006). HACCP: A systematic approach to food safety. Washington, D.C.: Food Products Association.
Schmidt, R. H., Goodrich, R. M., Archer, D. L., & Schneider, K. R. (2003). General overview of the causative agents of foodborne illness (FSHN033). Gainesville: Department of Food Science and Human Nutrition, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Retrieved June 29, 2007, from https://edis.ifas.ufl.edu/publication/FS099
Schmidt, R. H., and Newslow, D. L. (2007). Hazard analysis critical control points (HACCP) – Getting started, preliminary steps (FSHN0701). Gainesville: Department of Food Science and Human Nutrition, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Retrieved July 20, 2007, from https://edis.ifas.ufl.edu/fs137
Schmidt, R. H., and Newslow, D. L. (2007). Hazard analysis critical control points (HACCP) – Prerequisite programs (FSHN0702). Gainesville: Department of Food Science and Human Nutrition, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Retrieved December 8, 2022, from https://doi.org/10.32473/edis-fs138-2007
U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition. (2006). Foodborne pathogenic microorganisms and natural toxins handbook: The bad bug book. Retrieved December 8, 2022, from https://wayback.archive-it.org/7993/20170405001311/https:/www.fda.gov/Food/FoodborneIllnessContaminants/CausesOfIllnessBadBugBook/ucm296005.htm
U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition. (2001). Fish and fisheries products hazards and controls guidance, third edition. https://www.fda.gov/food/seafood-guidance-documents-regulatory-information/fish-and-fishery-products-hazards-and-controls [8 December 2022].
U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition. (2004). Juice HACCP hazards and controls guidance, first edition. Retrieved December 8, 2022, from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-juice-hazard-analysis-critical-control-point-hazards-and-controls-guidance-first
U.S. Department of Agriculture, Food Safety and Inspection Service. (2005). Meat and poultry hazards and controls guide. Retrieved December 8, 2022, from https://www.fsis.usda.gov/sites/default/files/import/Meat_and_Poultry_Hazards_Controls_Guide_10042005.pdf