Introduction, Host Range, and Distribution
Brown rot, caused by Monilinia spp., is one of the most economically harmful fungal diseases for peach and other stone fruit growers worldwide. Four Monilinia species have been found to cause brown rot. M. fructigena and M. laxa are two of the most common species found in Europe. Monilinia polystroma, an anamorphic species closely related to M. fructigena, has been described only in Japan. M. fructicola occurs in North and South America, Japan and much of Asia, Australia, and Europe (a more updated distribution can be found at https://gd.eppo.int/taxon/MONIFC/distribution). In North America, M. fructicola and M. laxa are the leading causal species of brown rot, blossom blight, and fruit rot in stone fruits (Martínez-García et al. 2013).
Brown rot fungus has the ability to attack blossoms, fruit, spurs (flower- and fruit-bearing twigs), and small branches under favorable conditions in the spring. Disease severity is dependent upon environmental conditions. Blossom blight can be expected in humid, rainy weather with mild daytime temperatures (68°F–77°F; 20°C–25°C) and cool nights. Mature fruit rot occurs at high temperatures in conjunction with high humidity. Under the right conditions, the entire tree's crop can be completely rotted. Fruit susceptibility fluctuates with the various stages of development; mature fruits are highly susceptible to disease, and fruit infection has the greatest impact on production (Figure 1).

Credit: D. Huff & A. Sarkhosh, UF/IFAS
Host Range and Distribution in Florida
Brown rot disease can occur on all stone fruit cultivars grown in Florida, including peaches, nectarines, and plums. Early-maturing varieties and natives can serve as sources of inoculum for later-maturing fruit cultivars (Bush et al. 2015).
Within the state of Florida, brown rot has historically been most problematic in the Panhandle and northernmost counties, with sporadic problems in central Florida. The likelihood of disease occurring each year and the severity of epidemics that occur generally decrease through the southernmost production areas. In Gainesville, the disease is common on dooryard peaches and plums but may occur after the early commercial harvest; however, when conditions are favorable for disease, losses can be severe.
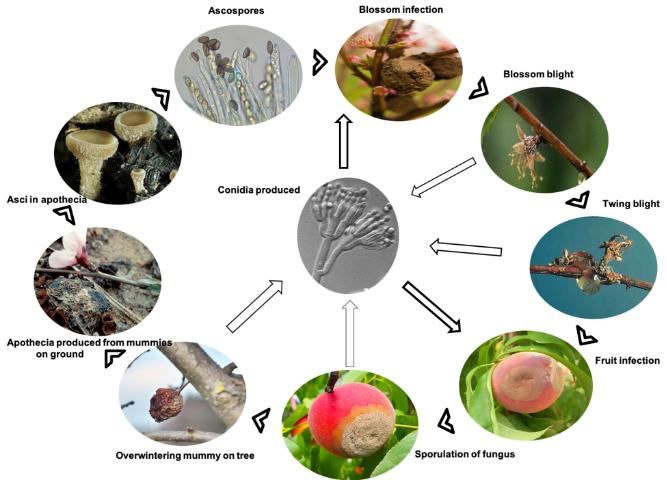
Disease Cycle
Overview
Primary Inoculum—starts the epidemic each season after a period of no disease activity
The most important sources of primary inoculum are mummified fruit on the ground that produce the sexual ascospores of the pathogen, and twig cankers that produce asexual conidia. These ascospores and conidia infect blossoms, causing a blossom blight that progresses into a twig blight and canker.
Secondary Inoculum—produced during the epidemic within a season, resulting in additional cycles of infection throughout the disease progression.
The most important sources of secondary inoculum early in the season include additional crops of conidia produced on the initially blighted blossoms and twigs. Conidia infect and also are produced on green fruit (usually wounded green fruit) and mature fruit later in the season. Conidia produced on fruit serve as inoculum for additional cycles of fruit and twig infections through the end of harvest as well as cycles of postharvest fruit rot during storage.
Infected fruit and twig cankers that remain in the field then restart the epidemic the following season.
Pathogen Survival
Brown rot fungus (M. fruticola) has the ability to overwinter on fruit mummies still attached to the tree, in infested crop debris on the ground surrounding the tree, and in twig cankers. Tiny mushroom structures (apothecia) may grow from the fruit mummies that have fallen to the ground and produce ascospores. Ascospores will generate for two weeks; afterward, the apothecia no longer contribute to the spread of infection. Apothecia may not be produced every year; however, conidia are. Conidia can arise on all overwintered, infected tissues, including fruit mummies and branch cankers. Spore formation of the fungus is favored by moderate temperatures (68°F–77°F; 20°C–25°C) and wet, humid weather.
Pathogen Dissemination
Spread of fungal spores to unaffected sites is carried out by wind and rainsplash. Insects, such as honeybees or beetles, may also transport the fungal inoculum to new infection sites. Early in the season, ascospores are carried primarily by wind from the fruit mummies at the soil level to the open or unopened blossoms and young shoots. Ascospores are forcibly discharged into the air by the slightest physical disturbance (Trial 2007).
Infection Process
The fungus requires free water to germinate and infect plant tissues. This water may come from rainfall, irrigation, or dew. Plant materials must remain wet for 3–5 hours to initiate fruit infection. Fruits that have been injured by insects, hail, or grower maintenance are more susceptible because these wounds allow direct access by the fungus to the fruit (Ellis 2018).
Infection can occur over a broad range of temperatures (32°F to 86°F; 0°C–30°C), with an optimum temperature range of 72°F to 77°F (22°C–25°C). However, if plant material remains wet for 24 hours or more, brown rot fungus infection is most likely to occur, regardless of the air temperature (Bush et al. 2015).
Wilting and powdery masses of secondary asexual spores called conidia will be observed on the flower shuck on infected blossoms. The infection can spread from the flower, into the spur, then the twig, where it will form a canker. Conidia will continue to be produced throughout the early summer from these cankers. Conidia can infect blossoms, green twigs, and green through fully ripe fruit.

Credit: P. Harmon, UF/IFAS
Credit: D. F. Ritchie, North Carolina State University
Credit: S. Shahkoomahally, UF/IFAS
Symptoms
Brown rot damage is easily observed on fruit, blossoms, and shoots. Infected blossoms will wilt and turn brown in color. Tan-gray tufts of masses of conidia will form on the outside of the flower shuck (Figure 5).

Credit: J. W. Pscheidt, Oregon State University
Infected shoots will develop sunken, brown, elliptical cankers that may become gooey in nature (gummosis). Cankers will typically form on new shoots as the disease progresses from infected blossoms. The canker has the potential to girdle and kill a shoot, resulting in twig blight. At this point, the leaves on the blighted twig will wilt and turn brown, but they can remain attached to the branch for a few weeks (Bush et al. 2015).
Young, immature fruits are susceptible to infection, but infections remain dormant, and symptoms do not typically develop until fruits near maturity. Fruits become more susceptible to infection as they mature. The first symptoms on mature fruit will appear as soft, brown spots that can quickly engulf the entire fruit (Figure 6). These spots will expand and produce spores (conidia) until covered in a powdery, tan mass. If allowed to come into contact with other plant material, these spores can cause the death of other branches (Beckerman 2015). Infected fruits will decay and shrivel until brown to black in color, either detaching from the tree or remaining attached (Biggs et al. 1995).

Credit: D. Huff, UF/IFAS

Credit: P. Harmon, UF/IFAS
Management
Cultural Control
Proper sanitation procedures are imperative to protecting the crop. Removal of fruit mummies (left intact on the tree or found on the ground) and other infected plant debris from the field will decrease the overwintering fungal inoculum. Sanitation measurements can be done from harvest in winter months to bloom. Mummies from any wild or neglected stone fruit trees adjacent to production fields should be removed as well (Obi et al. 2018).
Weak, dead, and cankered branches and twigs should be pruned throughout the winter and spring. Pruning the canopy to create more space between branches can help reduce inoculum by increasing sunlight and airflow. The use of microjet irrigation versus rain/overhead irrigation is recommended to avoid wetting fruits, flowers, branches, and twigs (Rungjindamai et al. 2014). Opening the canopy can improve chemical treatment coverage overall, which will show better results as well as use resources more effectively. Thinning fruits along the branch is extremely important because fruits making contact with each other show a higher incidence of infection due to increased contact with inoculum. Fruits that appear stunted should be thinned as well to remove another possible source of inoculum (Bush et al. 2015). Follow UF/IFAS recommendations to avoid excess nitrogen fertilizer, because too much nitrogen can lead to the greater susceptibility of the tree to disease (Byrne et al. 2012).

Credit: D. Huff, UF/IFAS
Chemical Control
Several fungicides are labeled for management of brown rot, and all must be applied before brown rot is found (Table 1).
Ideally, fungicide applications should begin at bloom and continue through harvest. Brown rot severity can vary from growing season to growing season, but when frequent rainfall is experienced during bloom and before harvest, disease is more likely and preventative treatments become more important to prevent fruit rot. In most years, and on farms that have had brown rot problems in the past, the most important applications of fungicides are made two weeks and again one week before harvest. If disease is active on the farm, fungicides may be needed at three weeks, two weeks, and one week before harvest and may need to be continued through harvest. It is exceptionally important to follow label instructions for all pesticides.
Fungicide resistance is a concern when managing brown rot. Populations of the pathogen in the Southeast have been documented to become resistant to several fungicide classes with site-specific modes of action (MOA). MOA classes are numbered and products with the same MOA code in Table 1 belong to the same group or class. Overapplication of products within a class can speed the development of resistance, resulting in the loss of that class as a disease management tool for brown rot on a farm or in a region. Current fungicide-resistance management recommendations include employing rotations of products from different site-specific classes and using tank mixes of multisite fungicide products with site-specific products.
The current status of fungicide resistance within populations of the brown rot pathogen in Florida is largely unknown; however, resistance is monitored in populations in the Carolinas and Georgia. The resistance information below was summarized from the Southeastern Peach, Nectarine, and Plum Management Guide (Blaauw et al. 2019).
Brown rot fungus is resistant to MOA code 1 fungicides (Topsin-M) and moderately resistant to MOA code 3 fungicides (propiconazole [Tilt], fenbuconazole [Indar], tebuconazole [Orius], and MOA code 3 generics). MOA code 3 fungicides are still important fungicides for brown rot control. Other important brown rot fungicides include the MOA code 11 fungicide azoxystrobin (Abound), as well as other code 11 fungicides in premixed combinations with MOA code 7 fungicides. Pristine, Merivon, and Luna Sensation contain different FRAC 7 and 11 actives. Premixes and the standalone MOA code 7 fungicide penthiopyrad (Fontelis) are highly effective options for brown rot management.
Management of Brown Rot in Organic Production
Due to the wet and humid climate in Florida, organic production of peaches in Florida is difficult. Also, because insect feeding can wound fruit and spread spores of brown rot fungi, brown rot management in organic production involves the integration of cultural practices, including orchard sanitation, pruning, thinning, and removing cankers and mummified fruits. Organic growers can use three applications of sulfur (lime sulfur) before the petals are open (at the pink stage), at petal drop, and at sepal drop, at seven-day intervals, respectively. Apply ½ to 1¼ gallons of product per 100 gallons of water. Do not apply more than 46 gallons of Lime Sulfur ULTRA product (136.5 lb of calcium polysulfide) per acre per year (https://orcalinc.com/assets/uploads/2018/11/Lime_Sulfur_ULTRA1e_Label.pdf).
References
Beckerman, J. 2015. Brown Rot on Tree Fruit in the Home Orchard. BP-45-W. West Lafeyette: Purdue University. https://www.extension.purdue.edu/extmedia/BP/BP-45-W.pdf
Biggs, A. R., K. D. Hickey, and K. S. Yoder. 1995. "Peach and Nectarine Brown Rot." In Mid-Atlantic Orchard Monitoring Guide, edited by H. W. Hogmire. NRAES-75. Ithaca, NY: Northeast Regional Agricultural Engineering Service, Cooperative Extension. https://hdl.handle.net/1813/67145
Blaauw, B., P. Brannen, D. Lockwood, G. Schnabel, and D. Ritchie (eds.). 2019. Southeastern Peach, Nectarine, and Plum Management Guide. Bulletin 1171. Athens, GA: University of Georgia College of Agricultural and Environmental Sciences. https://extension.uga.edu/publications/detail.html?number=B1171
Bush, E. A., K. S. Yoder, and A. H. Smith. 2015. Brown Rot on Peach and Other Stone Fruits. 450-721. Blacksburg: Virginia Cooperative Extension.
Byrne, D. H., M. B. Raseira, D. Bassi, M. C. Piagnani, K. Gasic, G. L. Reighard, M. A. Moreno, and S. Pe´rez. 2012. "Peach." In Fruit Breeding: Handbook of Plant Breeding, edited by M. L. Badenes, and D. H. Byrne. 505–570. Boston: Springer.
Diver, S., and T. Mumma. 2003. "Organic and Low-Spray Peach Production." IP047/35. Appropriate Technology Transfer for Rural Areas.
Ellis, M. A. 2018. Brown Rot of Stone Fruits. PLPATH-FRU-29. Columbus: The Ohio State University College of Food, Agricultural, and Environmental Sciences. https://ohioline.osu.edu/factsheet/plpath-fru-29
Martínez-García, P. J., D. E. Parfitt, R. M. Bostock, J. Fresnedo-Ramírez, A. Vazquez-Lobo, E. A. Ogundiwin, T. M. Gradziel, and C. H. Crisosto. 2013. "Application of Genomic and Quantitative Genetic Tools to Identify Candidate Resistance Genes for Brown Rot Resistance in Peach." PLOS ONE 8 (11): e78634. https://doi.org/10.1371/journal.pone.0078634
Obi, V. I., J. Barriuso, and Y. Gogorcena. 2018. "Peach Brown Rot: Still in Search of an Ideal Management Option." Agriculture 8: 125. https://doi.org/10.3390/agriculture8080125
Rungjindamai, N., P. Jeffries, and X. M. Xu. 2014. "Epidemiology and Management of Brown Rot on Stone Fruit Caused by Monilinia laxa." Eur. J. Plant Pathol. 140: 1–17. https://doi.org/10.1007/s10658-014-0452-3
Trial, F. 2007. "Fungal Cannons: Explosive Spore Discharge in the Ascomycota." FEMS Microbiology Letters. 276: 12–18. https://doi.org/10.1111/j.1574-6968.2007.00900.x
Ya´nez-Mendiza´bal,V., H. Zeriouh, I. Vin~a, R. Torres, J. Usall, A. deVicente, A. Pe´rez-Garci´a, and N. Teixido´. 2012. "Biological Control of Peach Brown Rot (Monilinia spp.) by Bacillus subtilis CPA-8 Is Based on Production of Fengycin-like Lipopeptides." Eur. J. Plant Pathol. 132: 609–619. https://doi.org/10.1007/s10658-011-9905-0