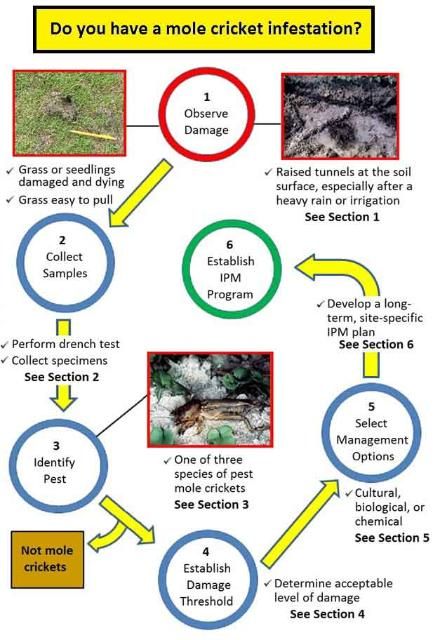
The purpose of this guide is to help people identify mole cricket infestations and manage them effectively and economically while minimizing environmental side effects. It can be used against pest mole crickets in sites such as landscapes, golf courses, athletic fields, sod farms, pastures, and vegetable fields. The first step in determining if you have a mole cricket problem at a site is to compare the existing damage to photographs of known mole cricket infestations. If the damage is likely caused by mole crickets, specimens should be obtained and the pest identified. You then must determine if the number of mole crickets is great enough to cause an unacceptable level of damage and decide what control measures should be used. Eventually, a long-term, sustainable integrated pest management (IPM) program must be established (Figure 1).
Section 1: Observe Damage
Plants Affected
Mole crickets are most often thought of as pests of grasses, such as bahiagrass, bermudagrass, centipedegrass, seashore paspalum, St. Augustinegrass, and zoysiagrass. However, other plants that can be damaged by mole crickets include but are not limited to beet, cabbage, cantaloupe, carrot, cauliflower, chrysanthemum, chufa, coleus, collard, eggplant, gypsophila, kale, lettuce, onion, peanut, pepper, potato, rice, spinach, strawberry, sugarcane, sweet potato, tobacco, tomato, and turnip (Worsham and Reed 1912).
Damage Caused
Mole cricket feeding and tunneling can damage or kill the affected plants, especially during warm and moist summer months when the nymphs are rapidly developing. Feeding on the underground plant parts can cause an overall decline, dead patches, and little to no root mass. In pastures, mole-cricket-infested grass may be uprooted by feeding livestock, rendering the grass unavailable for additional grazing. When mole crickets tunnel in the upper ten inches of the soil surface, plants can become dislodged or have limited water uptake. Moreover, tunneling can create raised surface ridges that disrupt ball roll on golf courses (Figure 2). It may be a symptom of mole cricket activity when plants appear drought-stricken even after sufficient irrigation (Figure 3). Vegetables and other plants are also affected through underground feeding on roots or tubers, and above-ground feeding on foliage or stems, along with their tunneling activity. Above-ground feeding often results in girdling around the base of the stem, or at times the entire plant may be chewed off and taken into a tunnel as food and consumed. This girdling is especially common in seedlings. Flying adult mole crickets are attracted to lights at night, and they often burrow into moist soil nearby to mate and lay eggs. An initial adult mole cricket infestation thus may be localized around outdoor light sources and/or sprinkler heads. After egg hatch and as the next-generation nymphs mature and disperse, greater areas become damaged.

Credit: N. Leppla, UF/IFAS

Credit: E. Buss, UF/IFAS
Section 2: Collect Samples
Sampling is a critical part of a well-designed IPM program; it is important to know which pests are present and roughly how many. A soap drench can bring mole cricket nymphs and adults to the soil surface, so their species and age can be determined. How many insects emerge from the soil may provide an idea of the extent of an infestation, but tunneling severity within a defined area may be more useful for decision-making. Below is a simple drench test for collecting specimens to be identified and for estimating mole cricket population densities. In this procedure, several 4 ft2 samples are taken from soil that must be moist:
- Mix ¾ oz. (1.5 tablespoons) of liquid dishwashing soap in a container with 1 gallon of water.
- Mark out a 2 ft. x 2 ft. area where mole cricket activity is suspected.
- Evenly pour the soap solution over the marked area.
- Observe the area for 3 minutes; count and collect the mole crickets that emerge.
- In many cases, control actions are justified if two or more mole crickets surface during the 3-minute sampling period. See Section 4, "Establishing Damage Threshold," for more information to help you determine whether to take action.
Section 3: Identify Pest
Three non-native pest species of mole crickets occur in Florida: the shortwinged mole cricket, Neoscapteriscus abbreviatus Scudder; the southern mole cricket, Neoscapteriscus borellii Giglio-Tos; and the tawny mole cricket, Neoscapteriscus vicinus Scudder. All three are believed to have been unintentionally transported into the southeastern United States around 1900. It is necessary to distinguish the native, non-pest mole cricket, genus Neocurtilla, from the invasive mole crickets in the genus Neoscapteriscus. Native mole crickets have four prominent dactyls (claws) on the forelegs and the pest mole crickets have two (Figure 4).

Credit: L. Buss, UF/IFAS

Credit: L. Buss, UF/IFAS
Mole Cricket Life Cycle
Eggs
(Figure 6): The female builds a circular egg chamber in the soil near one of the tunnels. The 3- to 4-cm-diameter chambers are placed 5–30 cm below the soil surface. Eggs are deposited in a cluster within the egg chamber, each mass containing 25–60 eggs. Eggs are gray to brownish and roughly oval, measuring about 3 mm long and 1.7 mm wide when fresh. Through the absorption of water, the eggs reach a final size of about 3.9 mm long and 2.8 mm wide. Egg development requires 10–40 days, depending on the soil temperature. A female produces 2–5 egg masses in a lifetime.

Credit: L. Buss, UF/IFAS
Nymphs
(Figure 7): Recently hatched nymphs, called first instars, are whitish but darken to their mature color during the first 24 hours. First instars may consume the egg shell or cannibalize siblings; however, they soon leave the egg chamber and burrow to the soil surface. Nymphs and adults are similar in appearance, except nymphs have underdeveloped external wings called wing-pads. Development time of nymphs varies, requiring 23–38 weeks during which they go through 8–10 instars before becoming adults.

Credit: J. Castner, UF/IFAS
Adults
(Figure 8): Adult mole crickets are light yellow to dark brown and measure 22–33 mm in length, depending on the species. They have enlarged forelegs with dactyls, blade-like projections used for digging. Their antennae are shorter than the body, and they have two long sensory appendages called “cerci” at the tip of the abdomen. Tawny and southern mole crickets become active at dusk when each male emits a “song” from its burrow that attracts a female of the same species. They mate within the burrow, after which the female may eject the male and occupy the burrow. Unlike the other two species, the shortwinged mole cricket male produces only a weak pulsing chirp that attracts a female.

Credit: L. Buss, UF/IFAS
Mole Cricket Seasonal and Geographic Distribution
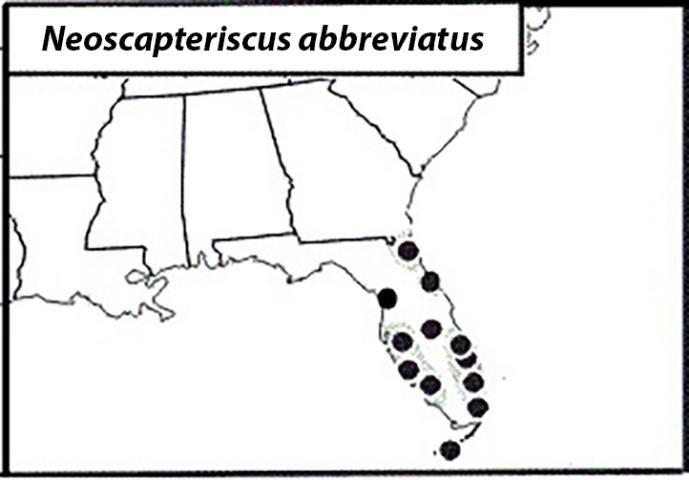
The Shortwinged Mole Cricket
The shortwinged mole cricket occurs mainly in coastal regions, with sandy soils (Figure 9). Because it is flightless, the species has not spread as extensively as the other two pest mole crickets. It currently has a limited geographical range in Florida, but all life-stages can occur year-round.
Credit: T. Walker, UF/IFAS
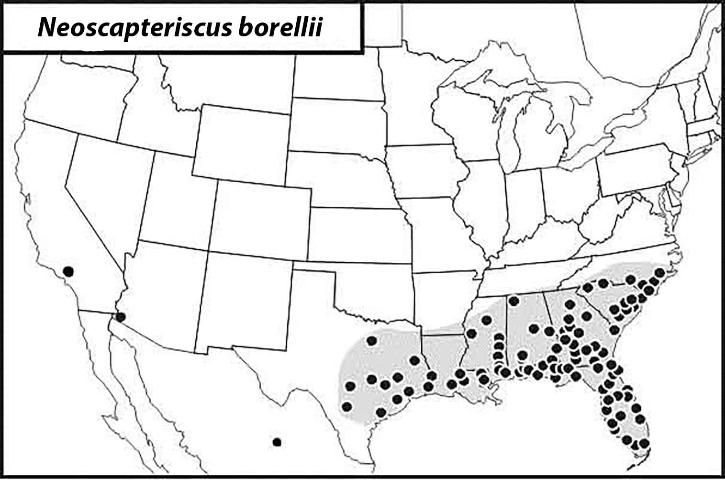
The Southern Mole Cricket
The southern mole cricket occurs across much of the southeastern United States from southern North Carolina to central Texas (Figure 10). It also has been reported in Yuma, Arizona, and Los Angeles County, California. It is distributed throughout Florida, occurring primarily in moist, sandy areas. This mole cricket usually has one generation per year, but it has two in southern Florida. Peak flights generally occur from April to June, with an additional minor flight around November. However, in south Florida, a second major flight usually occurs in July.
Credit: T. Walker, UF/IFAS
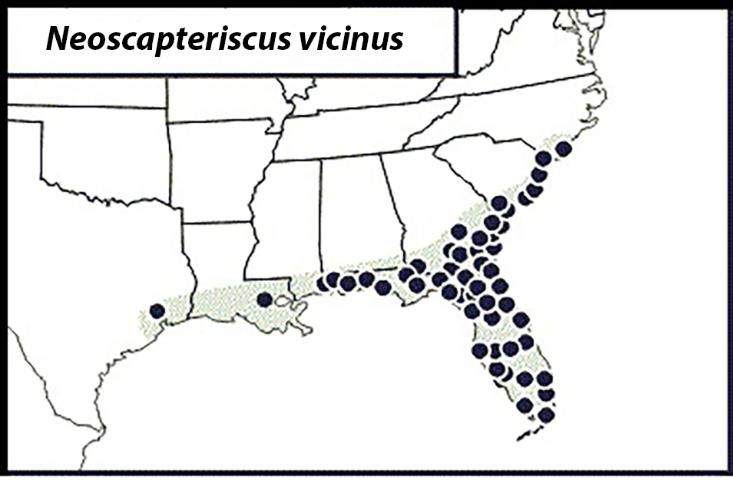
The Tawny Mole Cricket
The tawny mole cricket occurs within several miles of the Atlantic and Gulf coasts from North Carolina to eastern Texas (Figure 11). However, it is distributed throughout Florida and primarily inhabits well-drained, moist, sandy areas. This mole cricket has one full generation per year with a peak flight generally occurring in March–May, with an additional flight in the fall. Egg hatch occurs in April–June, after which nymphs develop for five months and become adults as early as September.
Credit: T. Walker, UF/IFAS
Section 4: Establish Damage Threshold
The amount of plant damage a homeowner or site manager determines as tolerable is called the "damage threshold." It varies with the site and expectations for plant quality. On athletic fields and golf courses, the more intensive management practices, lower cutting heights, and esthetic standards may dictate lower thresholds. In vegetable production, on the other hand, acceptable levels of damage may be low during the seedling stage but higher as the plants mature. Thresholds are highly subjective and vary with the condition of the plants.
The damage mole crickets cause is related to the species, stage, and number of mole crickets that infest the site. Tawny mole crickets, for instance, cause a relatively high degree of destruction, and a range of 2-4 adult mole crickets per 4 ft2 is a general upper limit warranting management action for turf, though most managers set the damage threshold somewhat higher for pastures. The plant damage nymphs cause increases as they grow and disperse. Continue sampling and re-evaluating thresholds throughout the mole crickets' life cycle to watch for increases both in the number of mole crickets and the damage they are causing. Ultimately, the severity of a mole cricket infestation and the associated damage threshold will dictate which control options will be most effective and economical.
Section 5: Select Management Options
Options for managing mole crickets in turfgrass include cultural, biological, and chemical control. Properly integrating several options will provide the greatest level of long-term control. After verifying the species, stage, and relative abundance of mole crickets, and deciding on a reasonable action threshold, select management practices from the following options.
Cultural Control
Cultural controls are steps taken in the management of a site that can make it less attractive or supportive for mole crickets. Steps may include selecting tolerant plant cultivars, altering soil moisture, reducing attractive lighting, and changing various growing practices. Cultural controls, such as lighting, may be implemented individually or used in conjunction with other methods.
Tolerant Cultivars
No turfgrass species or cultivar is completely resistant to mole cricket damage, although centipedegrass, St. Augustinegrass, and zoysiagrass are considered the least frequently injured. Bahiagrass, bermudagrass, and seashore paspalum tend to be the most susceptible to damage caused by mole crickets. Table 1 in the appendix describes some susceptible and tolerant turfgrass cultivars.
Soil Moisture
Soil moisture can affect mole crickets, significantly increasing plant damage at irrigated sites. Mole crickets remain closer to the soil surface when the soil is moist but tunnel deeper when the soil is dry. Rain after a long dry period causes an increase in the number of mole crickets in flight and may increase the number attracted to lights. During periods of egg-laying, females prefer to lay more eggs in irrigated areas than in non-irrigated ones. Egg survival decreases under drought conditions. Long-term control of soil moisture generally is not an option because it would disrupt plant growth, but the response of mole crickets to soil moisture can be used to time pest management practices. For example, insecticides could be more effective if applied after irrigation that brings mole crickets closer to the soil surface. Alternatively, flooding can drown the mole crickets or force them to move to higher ground where insecticides can be applied as spot treatments.
Lighting
Mole crickets fly at dusk for 1-2 hours during which they are attracted to light, especially ultraviolet and mercury-vapor lamps. To limit the incidence of mole crickets in turfgrass, lights should be turned off at a site during times of peak flight. Conversely, lights can be used to attract mole crickets for spot treatment with insecticides. If lights are necessary, yellow bulbs or filters can be used to minimize attraction of mole crickets.
Tillage
The objective of tilling is to expose mole crickets to predation or desiccation and kill them mechanically. Feeding by birds may be promoted by tilling, for example. In addition to exposing or damaging the insects, tilling can destroy their burrows and cause them to relocate. Tilling generally is not used on turfgrasses but can be effective on agricultural sites. Till when eggs and young nymphs are present because these life stages are more palatable to birds and less able to resist desiccation, so they are more likely to be killed than adults.
Plant Health
The plant's health can affect its tolerance to damage by mole crickets. Maintaining proper fertilization, irrigation, and soil conditions is important. For turfgrasses, leaving sufficient shoot growth after mowing is important because cutting too close increases stress on the grass. Mowing height recommendations are given in Table 2 in the appendix. For pastures, overgrazing should be avoided as this can cause significant stress to the grass.
Record Keeping
Areas that historically have been infested by mole crickets are likely to be re-infested. It therefore is important to document and map these preferred mole cricket habitats. Monitor these areas intensively so that you can implement control measures quickly before damage thresholds are exceeded.
Biological Control
Biological control is the use of living natural enemies to control pests. Natural enemies can be predators, parasites, pathogens, or competitors. Populations of some natural enemies may be enhanced by habitat manipulation. In some cases, natural enemies can be produced in large quantities and released at sites that have too few established natural enemies to effectively limit pest populations, keeping them below the damage thresholds. For pest mole crickets in Florida, widespread applications have been made of the entomopathogenic mole cricket nematode, Steinernema scapterisci, in addition to releases of the Larra wasp, Larra bicolor, and Brazilian red-eyed fly, Ormia depleta. These non-native natural enemies were imported, tested for safety and released by the UF/IFAS Mole Cricket Biological Control Program (Mhina et al. 2016). All are currently present in Florida, but none are available commercially. Specifics on the importation and introduction of these three natural enemies are described by Frank and Walker (2006).
Mole Cricket Nematode
This nematode (Figure 12) was introduced from South America and widely applied across Florida as a biopesticide until 2012. It infects large nymphs and adults, reproducing inside them to yield additional generations of nematodes. These parasites are not normally observed outside the host; they are spread throughout an area by the infected mole crickets.

Credit: L. Buss, UF/IFAS
Larra Wasp
This wasp (Figures 13 and 14) was introduced from South America into south Florida in 1981, and again into north Florida in 1988, to control pest mole crickets. It parasitizes only Neoscapteriscus spp. and does not sting people, so it was safe to release. The wasp is black with a red abdomen, and its wings are clear to smoky dark blue. A female usually lays one egg on each mole cricket it finds. The egg hatches in 6–7 days, the larva feeds on the mole cricket for 10–11 days and kills it, then pupates in a cocoon in the soil. A new adult emerges roughly 6 weeks later during the warmer months, but those that pupate in the fall may not become adults until the following Spring. Larra wasps lay eggs only on mole cricket adults and medium to large nymphs.

Credit: L. Buss, UF/IFAS

Credit: L. Buss, UF/IFAS
Larra wasps require a nectar source for their survival. The shrubby false buttonweed, Spermacoce verticillata (a.k.a. larraflower), is the preferred nectar source (Figure 15). White flowered pentas, Pentas lanceolata, and partridge pea, Chamaechrista fasciculata, are alternative nectar sources. If either of these plants or other nectar sources are available, larra wasps will appear and forage at least 200 yards from them to locate mole crickets. Larraflower can be invasive, so it should be contained. Partridge pea may be toxic if consumed by livestock.

Credit: L. Buss, UF/IFAS
Distribution
By the end of 2008, the larra wasp had spread into much of north and central Florida and had penetrated into parts of south Florida (Figure 16). It also expanded its range into southern and eastern Georgia and coastal areas of Alabama and Mississippi. More recently it has been reported from eastern South Carolina and southeastern North Carolina. In northern Florida, larra wasp adults are active from late April until the first hard frost; in southern Florida, activity may persist year-round, offering even greater mole cricket suppression.

Credit: J. H. Frank, UF/IFAS
Brazilian Red-Eyed Fly
This tachinid fly was introduced from South America to suppress invasive mole crickets. The Brazilian red-eyed fly is distributed in the southern and central parts of Florida with the northern boundary reaching Alachua County (Figure 17). The fly parasitizes a pest mole cricket adult by depositing a larva nearby, the larva finds the adult, develops inside it, and kills it. Golf courses inhabited by the Brazilian red-eyed fly have considerably less damage than those without the fly.
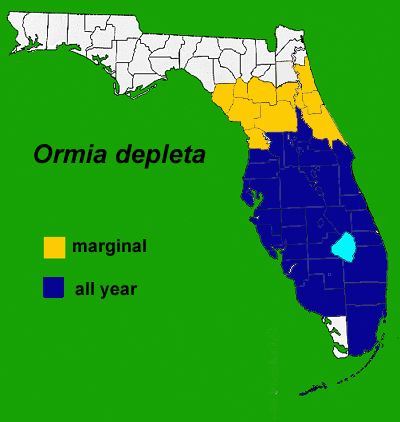
Credit: J. H. Frank, UF/IFAS

Credit: L. Buss, UF

Credit: L. Buss, UF
Mole Cricket Predators
Naturally occurring predators of mole crickets include raccoons, opossums, armadillos, birds, spiders, tiger beetles, and many other insectivorous animals. Unfortunately, foraging by some of these predators, especially armadillos, can cause considerable damage to turfgrass.
Chemical Control
Mole cricket IPM includes the use of insecticides when necessary; however, applications can be expensive and disruptive to biological control. Apply an insecticide only when the plant damage threshold is met or exceeded, and follow the instructions on the label. Time applications and target them to infested areas, thus reducing costs and environmental risks. On golf courses, for example, it’s frequently most effective to apply insecticides only to fairways, greens, and tees, leaving roughs and driving ranges untreated to maintain populations of beneficial organisms. Small mole cricket nymphs feeding and growing during the summer months are more susceptible to insecticides than large nymphs present in late summer and fall.
Table 3 lists selected insecticide active ingredients for products that are currently registered for use in Florida on pest mole crickets in residential lawns, golf courses and athletic fields, pastures, vegetables, and sod farms. To minimize resistance to insecticides, active ingredients should be rotated based on the Insecticide Resistance Action Committee (IRAC) group numbers. The table and associated appendix in this publication serve as guides only: keep in mind that the information in them can become outdated if insecticide registrations change.
The appendix includes Table 5that lists registered insecticide products formulated with the active ingredients listed in Table 3. Restricted-use insecticides are to be applied by a licensed applicator. You must read and understand the current product label before applying any insecticide. The label lists all specific sites and pests for which an insecticide may be applied legally. It also displays a signal word indicating the relative toxicity of the product to mammals: slightly toxic (CAUTION), moderately toxic (WARNING), or highly toxic (DANGER).
Section 6: Establish IPM Program
Develop a long-term, site-specific IPM program by combining cultural, biological, and chemical control measures to suppress pest mole crickets to levels that assure plant damage thresholds are not exceeded and that minimize costs and risks to humans and the environment. The program is based on plant selection and growing practices and mole cricket biology and management options.
The following are guidelines for developing an IPM program for turfgrass:
- Use a tolerant grass cultivar or species, such as centipedegrass or zoysiagrass.
- Maintain healthy grass with proper irrigation and cutting.
- Perform routine soil testing and add fertilizer or lime as needed.
- Reduce watering during winter months; mole crickets require moist soil.
- Plant a nectar source, such as larraflower or partridge pea, to attract and support Larra wasp populations.
- Eliminate lights from sunset to well past dark during months of peak mole cricket flight.
- Sample regularly for mole crickets; 2–4 per 4 ft2 may require management.
- Apply insecticides at infested sites only if plant damage thresholds are exceeded; evaluate their effectiveness.
- Target and map areas that become infested.
- Rotate insecticide active ingredients (IRAC numbers and letters) to delay pesticide resistance.
Acknowledgments
This project was supported by USDA, National Institute of Food and Agriculture, CPPM, IPM grant No. 2017-70006-27149 and the Southern IPM Center.
Selected References
Abraham C. M., Held D. W., and Wheeler C. 2010. Seasonal and diurnal activity of Larra bicolor (Hymenoptera: Crabronidae) and potential ornamental plants as nectar sources. Applied Turfgrass Science. https://doi.org/10.1094/ATS-2010-0312-01-RS
Anonymous. 2021. Pest Mole Crickets and Their Control. UF/IFAS Department of Entomology and Nematology. http://entnemdept.ufl.edu/pestmolecrickets/index.htm (June 2021)
Braman S. K., Duncan R. R., Hanna W. W., and Hudson W. G. 2000. Evaluation of turfgrasses for resistance to mole crickets (Orthoptera: Gryllotalpidae). HortScience 35:665–668. https://doi.org/10.21273/HORTSCI.35.4.665
Braman S. K., Pendley A. F., Carrow R. N., and Engelke M. C. 1994. Potential resistance in zoysiagrasses to tawny mole crickets (Orthoptera: Gryllotalpidae). Florida Entomologist 77:301–305. https://doi.org/10.2307/3496099
Capinera J. L. and Leppla N. C. 2001. Scapteriscus abbreviatus Scudder (Insecta: Orthoptera: Gryllotalpidae). Featured Creatures, UF/IFAS Entomology and Nematology Department. http://entnemdept.ufl.edu/creatures/orn/turf/pest_mole_crickets.htm (June 2021)
Capinera J. L. and Leppla N. C. 2007. Shortwinged Mole Cricket, Neoscapteriscus abbreviatus Scudder; Southern Mole Cricket, Neoscapteriscus borellii Giglio-Tos; and Tawny Mole Cricket, Neoscapteriscus vicinus Scudder (Insecta: Orthoptera: Gryllotalpidae). EENY-235. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/in391 (June 2021)
Chong J. 2009. Comparative efficacy of neonicotinoids and selected insecticides in suppressing tunneling activity of mole crickets (Orthoptera: Gryllotalpidae) in turfgrass. Journal of Agricultural and Urban Entomology 26:135–146. https://doi.org/10.3954/1523-5475-26.3.135
Dillman, A. R., Cronin, C. J., Tang, J., Gray, D. A., and Sternberg, P. W. 2014. A modified mole cricket lure and description of Scapteriscus borellii (Orthoptera: Gryllotalpidae) range expansion and calling song in California. Environmental Entomology 43:146-156. https://doi.org/10.1603/EN13152
Frank J. H. and Parkman J. P. 1999. Integrated pest management of pest mole crickets with emphasis on the southeastern USA. Integrated Pest Management Review 4:39–52. https://doi.org/10.1023/A:1009628915989
Frank J. H. and Walker T. J. 2006. Permanent control of pest mole crickets (Orthoptera: Gryllotalpidae: Scapteriscus) in Florida. American Entomologist 52:138–144. https://doi.org/10.1093/ae/52.3.138
Frank J. H., Walker T. J., and Parkman J. P. 1996. The introduction, establishment and spread of Ormia depleta in Florida. Biological Control 6: 368–377. https://doi.org/10.1016/B978-012265040-6/50001-1
Held, D. W. and Xu, Y. 2015. Field Performance and Consumption of Indoxacarb Bait for Control of Mole Crickets (Scapteriscus spp.) in Turfgrass. Crop, Forage & Turfgrass Management 1:1-6. https://doi.org/10.2134/cftm2015.0132
Hertl, P. T. and Brandenburg, R. L. 1996. Control of Mole Crickets on Golf Course Fairways in North Carolina, 1995. Arthropod Management Tests 21:333-334. https://doi.org/10.1093/amt/21.1.333a
Hertl P. T. and Brandenburg R. L. 2002. Effect of soil moisture and time of year on mole cricket (Orthoptera: Gryllotalpidae) surface tunneling. Environmental Entomology 31:476–481.
Hertl P. T. and Brandenburg R. L. 2013. First record of Larra bicolor (Hymenoptera: Crabronidae) in North Carolina. Florida Entomologist 96:1175–1176.
Kostromytska O. S., Buss E. A., and Scharf M. E. 2011. Toxicity and neurophysiological effects of selected insecticides on the mole cricket, Scapteriscus vicinus (Orthoptera: Gryllotalpidae). Pesticide Biochemistry and Physiology 100:27–34. https://doi.org/10.1016/j.pestbp.2011.01.012
Kostromytska, O. S., Scharf, M. E., and Buss, E. A. 2018. Behavioral responses of pest mole crickets, Neoscapteriscus spp.(Orthoptera: Gryllotalpidae), to selected insecticides. Pest Management Science 74:547-556. https://doi.org/10.1002/ps.4732
Mhina, G. J, Leppla, N. C. Thomas, M. H., and Solís, D. 2016. Cost effectiveness of biological control of invasive mole crickets in Florida pastures. Biological Control 100:108–115. https://doi.org/10.1016/j.biocontrol.2016.05.017
Parkman J. P., Frank J. H., Walker T. J., and Schuster D. J. 1996. Classical biological control of Scapteriscus spp. (Orthoptera: Gryllotalpidae) in Florida. Environmental Entomology 25:1415–1420. https://doi.org/10.1093/ee/25.6.1415
Portman S. L., Frank J. H., McSorley R., and Leppla, N. C. 2010. Nectar-seeking and host-seeking by Larra bicolor (Hymenoptera: Crabronidae), a parasitoid of Scapteriscus mole crickets (Orthoptera: Gryllotalpidae). Environmental Entomology 39:939–943. https://doi.org/10.1603/EN09268
Reinert, J. A. 1996. Late season control of mole crickets on high maintenance turf in Texas, 1995. Arthropod Management Tests 21:334-334. https://doi.org/10.1093/amt/21.1.334
Reinert J. A. and Busey P. 2001. Host resistance to tawny mole cricket, Scapteriscus vicinus, in Bermudagrass, Cynodon spp. International Turfgrass Society Research Journal 9:793–797.
Rohde, B. B., Allen, P. E., Benda, N., Brun, A., Mankin, R. W., and Dale, A. G. 2019. An acoustic trap to survey and capture two Neoscapteriscus species. Florida Entomologist 102:654-657. https://doi.org/10.1653/024.102.0316
Thompson, S. R. and Brandenburg, R. L. 2005. Tunneling responses of mole crickets (Orthoptera: Gryllotalpidae) to the entomopathogenic fungus, Beauveria bassiana. Environmental Entomology 34:140-147. https://doi.org/10.1603/0046-225X-34.1.140
Trenholm, L. E., Unruh, J. B., and Cisar, J. L. 2001. Mowing Your Florida Lawn. ENH-10. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/LH028 (June 2021)
Ulagaraj S. M. 1975. Mole crickets: ecology, behavior, and dispersal flight (Orthoptera: Gryllotalpidae: Scapteriscus). Environmental Entomology 4:265–273. https://doi.org/10.1093/ee/4.2.265
Walker T. J. and Moore T. E. 2013. Singing insects of North America. http://entnemdept.ifas.ufl.edu/walker/Buzz/ (June 2021)
Walker T. J., Reinert J. A., and Schuster D. J. 1983. Geographical variation in flights of mole crickets, Scapteriscus spp. (Orthoptera: Gryllotalpidae). Annals of the Entomological Society of America 76: 507–517. https://doi.org/10.1093/aesa/76.3.507
Worsham, E. L. and Reed, W. V. 1912. The Mole Cricket: Scapteriscus didactylus Latr. Georgia Experiment Station Bulletin No. 101: 251–263.
Appendix
The applicator holds full responsibility in verifying the legal usage and assumes all associated liability when applying any pesticide. Before applying an insecticide, verify that your target pest and specific site of application are authorized by consulting the product’s label and always wear proper personal protective equipment.
Tables
Table 3. Selected insecticides for controlling mole crickets (compiled by N. C. Leppla).
|
Active ingredient products | IRAC Group | Application range total/yr | Reentry interval |
Applications product details | |
|
Acephate Orthene | 1B |
GC, 1-2 oz/gal. S, 1 1/3-1 ½ oz/gal. | 24 h |
GC, S allow 3 d to harvest | |
|
Bifenthrin Talstar | 3A |
0.5-1.0 oz/1000 ft2 Up to 10 gal/1000 ft2 | 12 h |
RL, GC, V, S water in | |
| 1A |
RL, 0.75-0.9 lbs/1000 ft2, 4 appl./yr | Dry surface |
RL, GC, P, V, S | |
|
Chlorpyrifos Lorsban, Dursban | 1B |
1.5 oz/1000 ft2 2 lbs AI/acre/yr | 24 h |
GC, S Apply to wet surface | |
|
Clothianidin Arena | 4A |
0.29 oz (1-5 gal)/1000 ft2, 0.4 lb/acre/yr | Dry surface |
RL, S Apply to early instars | |
|
Fipronil Chipco Choice | 2B | 4.6-9.4 oz/1000 ft2 50 lbs/yr | 24 h |
RL, GC, S avoid water bodies | |
| RL= Residential lawns, GC= Golf courses and athletic fields, P=Pastures, V=Vegetables, S=Sod farm. | |||||
Mole cricket stages and partial IPM program for North Central Florida.
Insecticide active ingredients and products registered for controlling mole crickets at selected sites in Florida.
Active ingredients contained in insecticide products registered for golf courses and athletic fields.