The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Most pest species of subterranean termites in North America belong to the endemic genus Reticulitermes which, as a group, are often referred to as “native subterranean termites.” All Reticulitermes species in the United States can be found associated with dead trees and rotting wood in natural habitats (Figure 1), but also have the ability to feed on structural wood material. Reticulitermes species are found in every state in the continental United States except Alaska but are most common in the warm and humid southeastern region. While the invasive Formosan subterranean termite, Coptotermes formosanus Shiraki (EENY121), has a distribution primarily restricted to the subtropical climate of the southeastern United States, endemic Reticulitermes species are distributed across temperate climates (Janowiecki et al. 2021). Reticulitermes species most often associated with structures in the United States are within these five following species.
- The eastern subterranean termite, Reticulitermes flavipes (Kollar), is the most widely distributed species and is found in the entire eastern region of North America as far north as Ontario, Canada, and south to Key Largo, Florida. It has a pest status across its native range but is also a pest species with an invasive status in France, Chile, and Spain (Eyer et al. 2021). It was also found in two locations in California.
- The western subterranean termite, Reticulitermes hesperus Banks, is found along the entire Pacific Coast ranging from southern California to British Columbia.
- The arid-land subterranean termite, Reticulitermes tibialis Banks, occurs in the inter-mountain region of the West.
- The dark southern subterranean termite, Reticulitermes virginicus (Banks), is distributed in the southeastern United States.
- The light southern subterranean termite, Reticulitermes hageni Banks, is also distributed in the southeastern United States.

Credit: Thomas Chouvenc, UF/IFAS
In Florida, Reticulitermes flavipes and Reticulitermes virginicus are both found statewide (Figure 2). In comparison, Reticulitermes hageni is not observed as often as the two other Reticulitermes species found in Florida (Scheffrahn et al. 1988). Of the $20 billion annually spent for termite control and repairs in the United States, subterranean termites account for 80% share, and the majority of this is attributed to Reticulitermes flavipes, Reticulitermes virginicus, Reticulitermes hesperus, and C. formosanus (Rust and Su 2012).
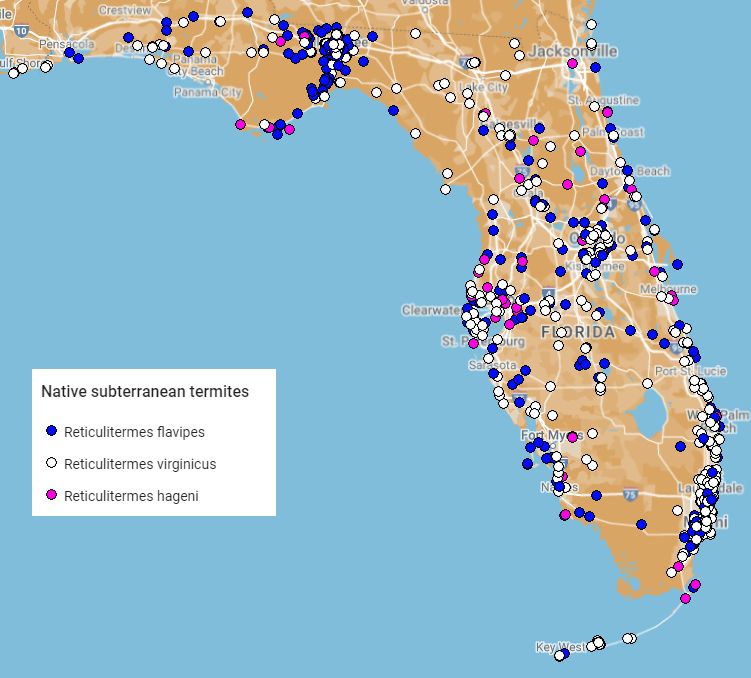
Credit: Thomas Chouvenc, Rudolf Scheffrahn UF/IFAS
Pest Status and Detection in Structures
Because termites consume cellulose (the main structural components of plant cells), any wood material in a house is a potential food source. Termites may also damage non-wood material while foraging for food. However, due to their cryptic nature, structural infestations by a subterranean termite colony are usually not readily visible. Damage to structural wood from Reticulitermes sp. may be observed during house remodeling (Figure 3A), or if signs of foraging activity become obvious, such as the construction of shelter tubes (=mud tubes) from the ground up (Figure 3B). Subterranean termite colonies form a network of interconnected feeding sites beneath or above the soil surface. A single colony of subterranean termites, especially those of Reticulitermes flavipes, may contain 100,000–1,000,000 termites and forage up to 150ft in search of food (Su et al. 1993). When subterranean termites search for food aboveground, they may enter a house through small cracks or joints in the foundation or by building shelter tubes along the foundation wall. These tubes are the equivalent of highways connecting the underground termite population with aboveground food sources.
Most often, residents of an infested structure may become aware of the infestation when annual dispersal flights of winged termites (called alates) occur, as hundreds to thousands of individuals can emerge during these events (Figure 3C). To inspect for active subterranean termite infestation or damage, wood material can be probed with a screwdriver or an equivalent to locate infested wood. The surface of severely damaged wood may appear blistered or peeling, as termites hollow out the wood leaving a paper-thin surface. If a structural infestation by a subterranean termite colony is suspected, an inspection should be performed by a licensed professional (see ENY2044).
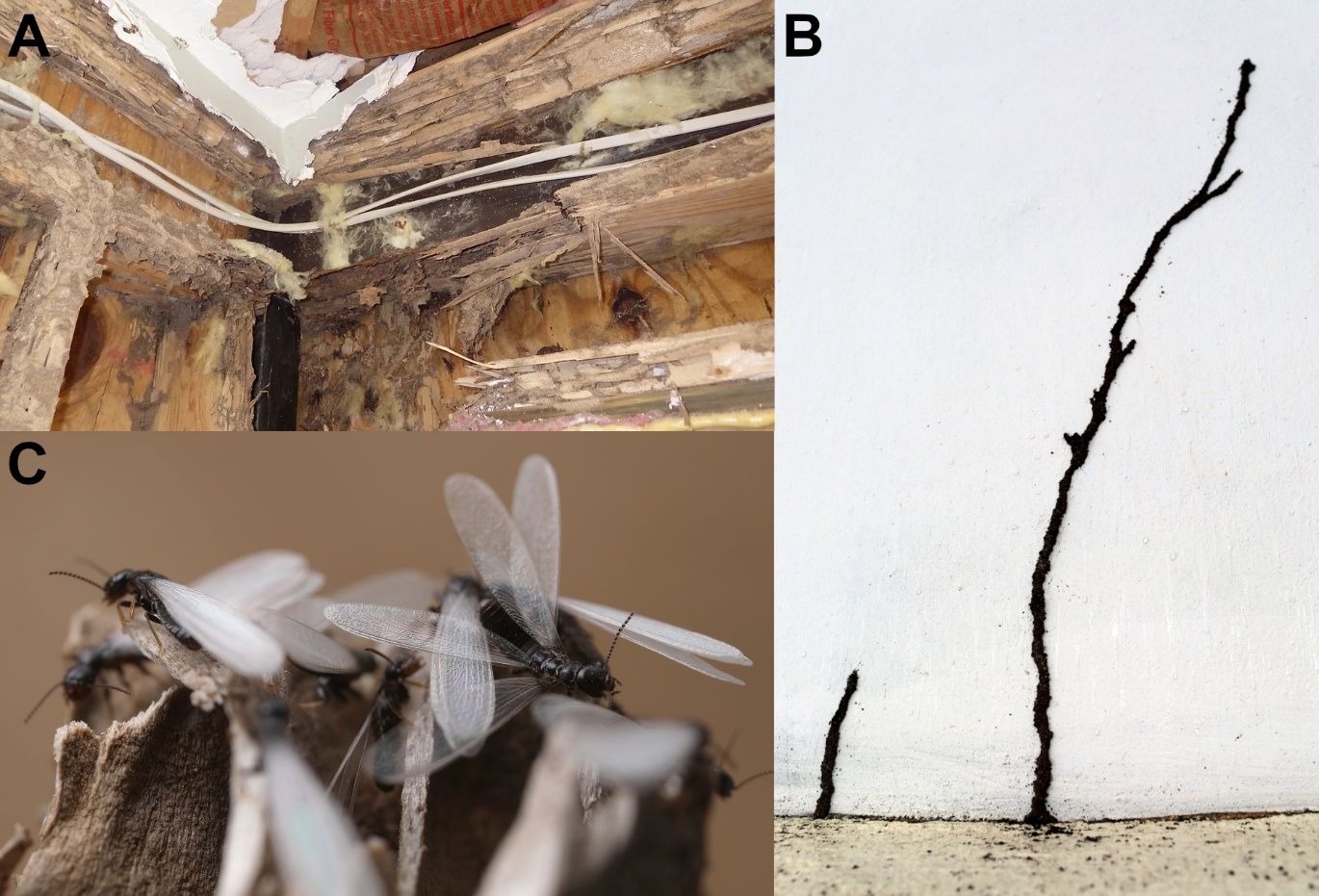
Credit: A) Rudolf Scheffrahn UF/IFAS; B and C) David Mora del Poso
Identification
From a pest management perspective, identifying Reticulitermes sp. samples as “native subterranean termites” is often sufficient, as protocols for management are similar for all Reticulitermes sp. However, it is necessary to distinguish them from other subterranean termites found in Florida (Coptotermes, Heterotermes, Prorhinotermes, see ENY2079), as detection and management approaches may vary. As with other termite species, Reticulitermes colonies contain three primary castes; the reproductives (king, queen, alates, nymphs, and supplementary reproductives), soldiers, and workers (see life colony life cycle below). Alates and soldiers are the two primary castes used for species identification for all termite species in Florida (Scheffrahn and Su 1994).
Alates
Reticulitermes alates can be identified by wing characters which separate them from all other termite genera in Florida. First, like all other subterranean termites (Rhinotermitidae), wings possess two sclerotized veins (costal margin and radial sector) along the entire front end (Figure 4, arrows). Second, wings are not covered with hair and the median and cubitus veins are present within the front-central part of the wing. Finally, the wing displays a nonpigmented “reticulated” texture (Figure 4).
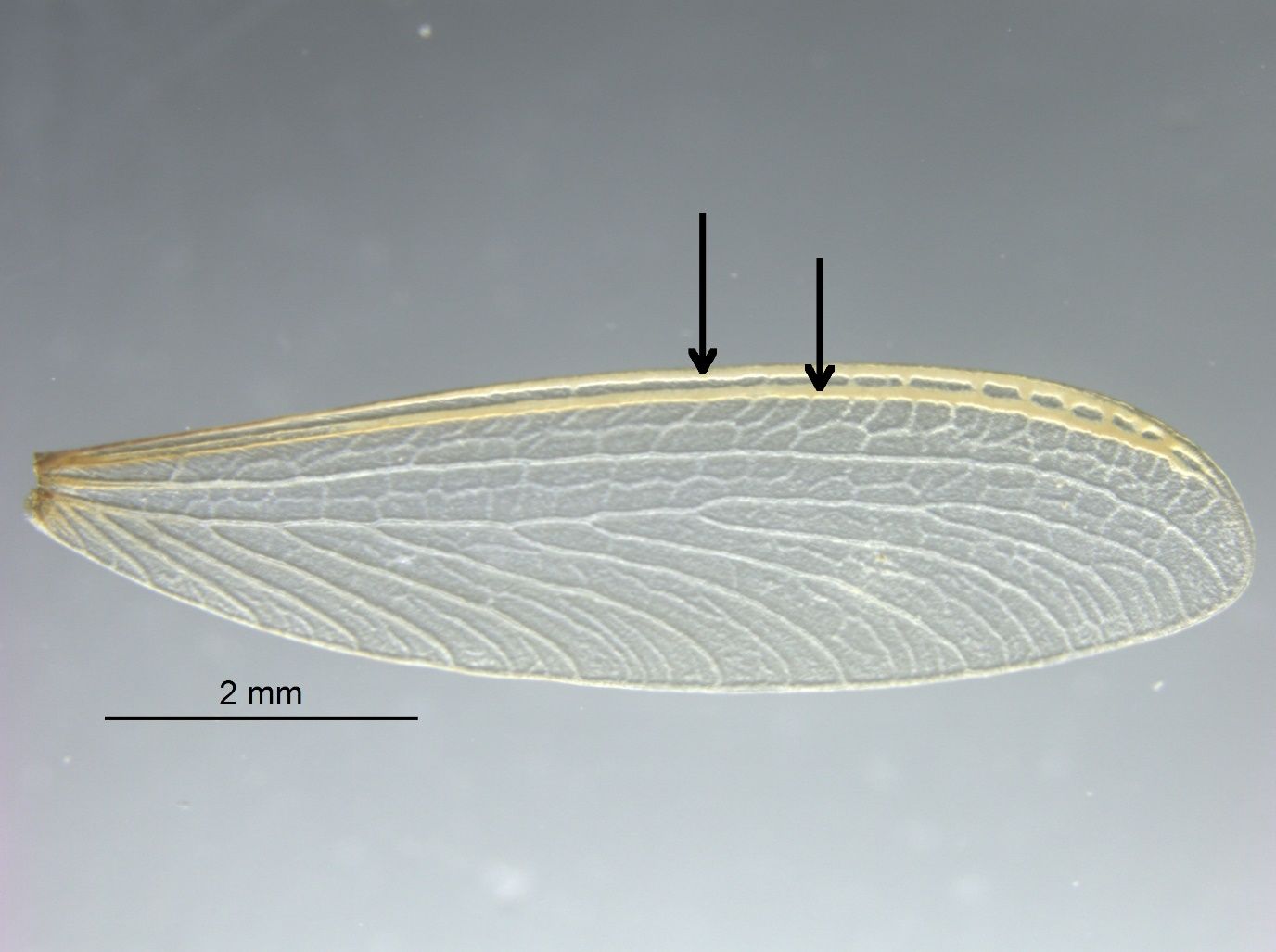
Credit: Rudolf H. Scheffrahn, UF/IFAS
The alates of Reticulitermes flavipes and Reticulitermes virginicus are dark brown (almost black to the naked eye), while those of Reticulitermes hageni are yellowish brown (Figure 5). Alates of Reticulitermes flavipes are generally larger (approximately 0.4" long including wings) than those of Reticulitermes virginicus or Reticulitermes hageni (approximately 0.3" long) (Figure 5A). However, the anterolateral pronotum edge is rounded in Reticulitermes virginicus, while relatively angular in Reticulitermes flavipes (Figure 5A). Furthermore, in alates of Reticulitermes flavipes, the diameter of the ocellus is smaller than the distance between the ocellus and the compound eye, while in alates of Reticulitermes virginicus, the diameter of the ocellus is equal or wider than the distance between the ocellus and the compound eye (Figure 5B). After indoor flights, most Reticulitermes sp. alates are found dead (dried up) near windows or in sinks and bathtubs—usually with their wings still attached.
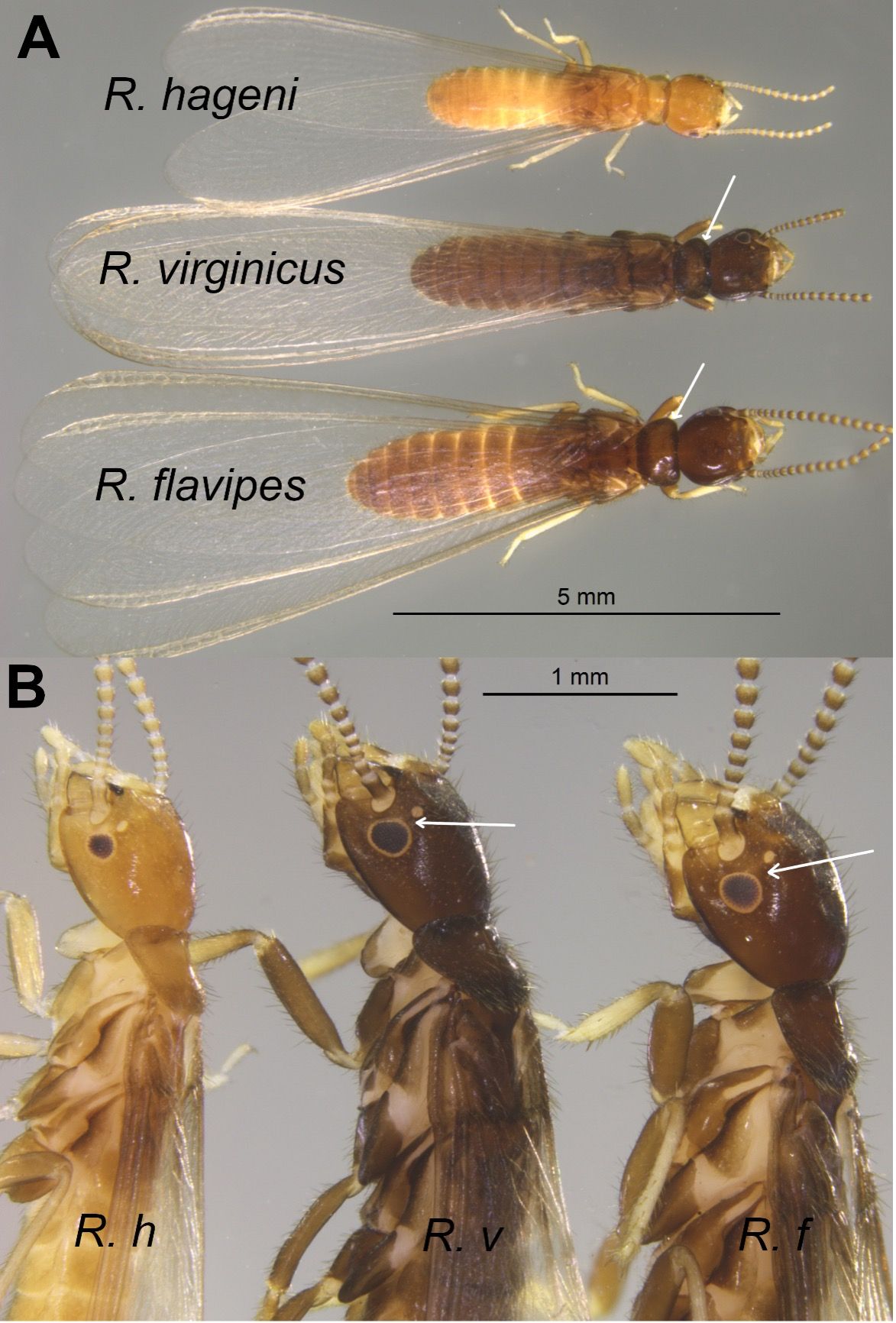
Credit: Rudolf H. Scheffrahn, UF/IFAS
Soldiers
In Florida, Reticulitermes sp. soldiers are recognized by the combination of three primary morphological characters. First, the soldiers of all Florida subterranean termites (Rhinotermitidae) are distinguished from those of drywood or dampwood termites (Kalotermitidae) by their relatively smaller size and the relative width of the pronotum. In subterranean termites, the pronotum (segment immediately behind the head) is narrower than the head width, while in Kalotermitidae, the pronotum is equally or wider than the head. Second, when comparing Florida subterranean termite genera, Reticulitermes spp. soldiers display a rectangular-shaped head while Coptotermes sp. soldiers (EENY128) and Prorhinotermes soldiers (EENY282) display an oval-shaped head (Figure 6). Third, Heterotermes sp. soldiers also display a rectangular-shaped head, but the mandibles in Reticulitermes sp. soldiers are curved at the tip in an S shape, while mandible curvature in Heterotermes sp. is less pronounced (EENY127). In addition to morphological difference, the soldier ratio and soldier behavior differ among subterranean genera. When conducting an inspection for live subterranean termite activity, if many soldiers accumulate at the intrusion point, it most likely is Coptotermes sp. (~10% soldiers in the colony), and if little to no soldiers can be found, it most likely is Reticulitermes sp. (1% to 2% soldiers in the colony).

Credit: Rudolf H. Scheffrahn, UF/IFAS
Life Cycle
Termites are eusocial insects belonging to the order Blattodea (cockroaches). They form colonies with an established reproductive division of labor (few individuals are in charge of reproduction), overlapping generations of individuals, and cooperative brood care. The queen(s) and king(s) (Figure 7) are in charge of reproduction, and the queen lays eggs that can develop into various castes, depending on the need of the colony. Workers are in charge of finding and digesting food, nest maintenance and brood care, while soldiers are in charge of colony defense. Nymphs are produced seasonally to become alates (winged adults) which fly away from the parental nest to establish new colonies. Reticulitermes possess a bifurcated development which implies that adult imagoes (alates) can only be produced through the nymphal pathway (Figure 8). Reticulitermes therefore have a “true” worker caste, which is contrary to drywood and dampwood termites, which have a linear development pathway, where pseudergates (false workers) have the potential to become alates. However, Reticulitermes can produce secondary reproductives (supplementary queens and kings) as some nymphs become reproductively active without completing their development toward the alate (=nymphoid reproductives). In addition, Reticulitermes can also produce a third form of reproductives via the worker development path (=ergatoid reproductives).

Credit: Lyle Buss, UF/IFAS
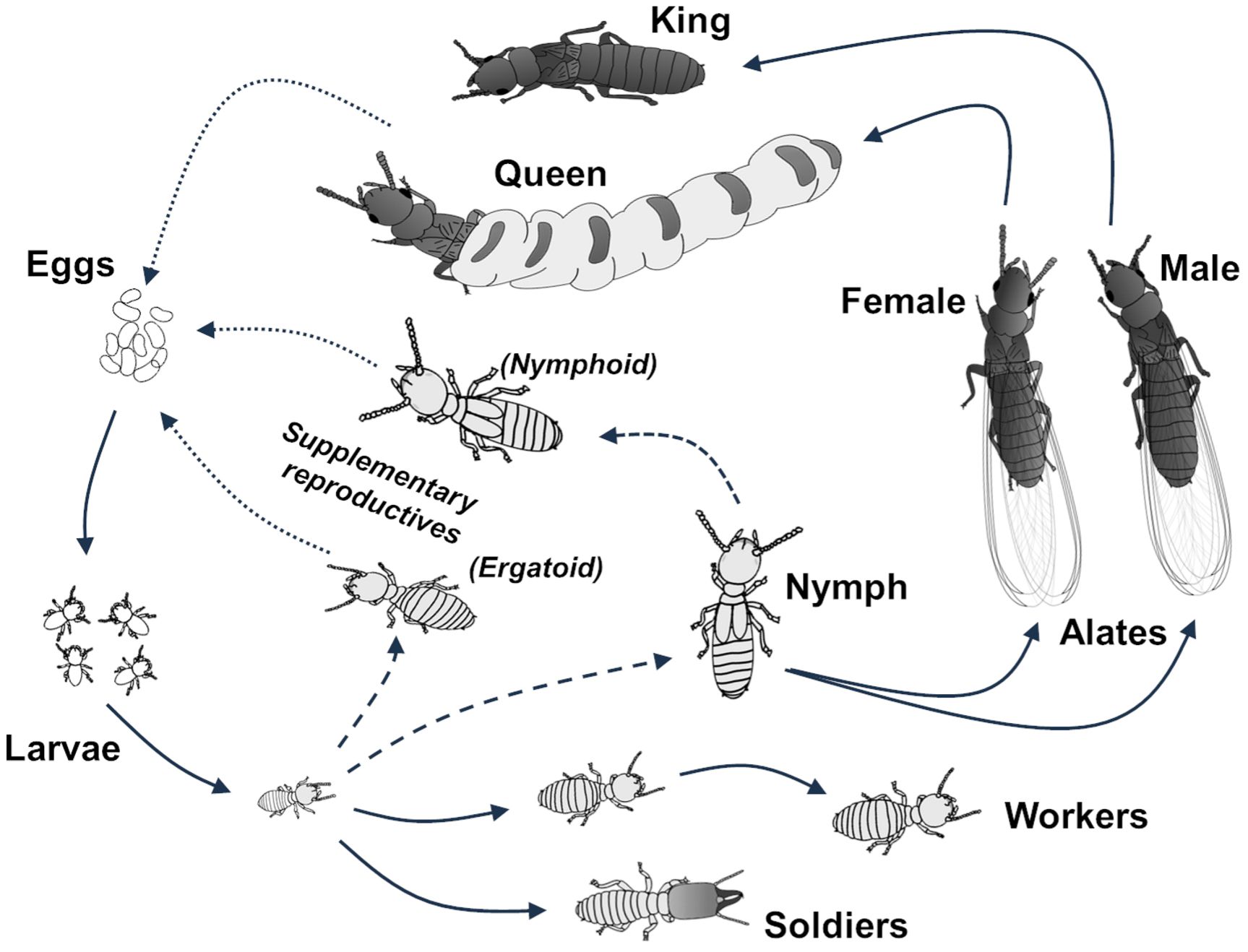
Credit: Thomas Chouvenc, UF/IFAS
Dispersal Flight Season
In Florida, established Reticulitermes sp. colonies feed on wood resources year-long. However, they engage in seasonal dispersal flights, which differ among the three Reticulitermes species in Florida (Figure 9). Reticulitermes hageni alate flights begin in early December and last until early February, while Reticulitermes flavipes flights start in early January and end in April. Dispersal flights of Reticulitermes virginicus occur between early February and late May. Dispersal by Reticulitermes flavipes and Reticulitermes virginicus occurs during warm, sunny, and windless early afternoons usually after rain, while Reticulitermes hageni alates disperse in the evening.
After a brief flight, alates drop to the ground and shed their wings. Females begin to search for potential nesting sites such as moist crevices with wood, and males follow closely behind in a tandem. The pair forms a royal chamber in a moist site near wood and the queen begins laying eggs, thus starting the life cycle of a subterranean termite colony. It may take five to 10 years for a newly founded colony to form a mature colony that produces alates.
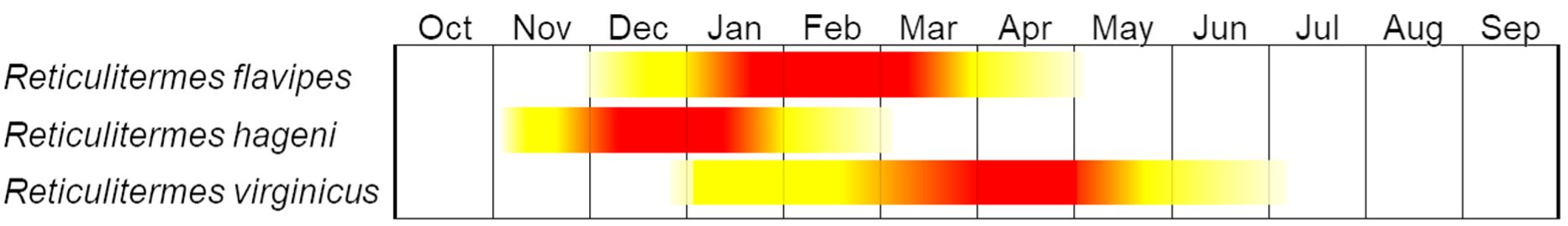
Credit: Rudolf H. Scheffrahn and Thomas Chouvenc, UF/IFAS
Management
Preventive Practices
Because subterranean termites forage in soil, it is important to keep structural lumber from direct contact with soil. Keeping the lower foundation walls and siding clear of vegetation or mulch makes it easier to inspect for termite shelter tubes. Subterranean termites need moisture for survival. Leaky plumbing, air conditioning condensate, and any portion of a building and its perimeter that collects excessive amounts of moisture should be corrected to maintain an environment that is less favorable for subterranean termite activity.
Termite Management
Currently in the US, there are two primary ways to manage subterranean termite populations: 1) non-repellent liquid termiticides and 2) chitin-synthesis inhibitor (CSI) baits. Both methods are widely used in the US but use different modes of action and different approaches (see ENY2044 for further details). Liquid termiticides treatments prevent the subterranean termite colonies from accessing the protected structure, while CSI baits eliminate a colony that feeds on the bait (Su 2005, Chouvenc 2018, Shults et al. 2021). Subterranean termite treatments must be applied by a licensed pest management provider.
Selected References
Chouvenc T. 2018. Comparative impact of chitin synthesis inhibitor baits and non-repellent liquid termiticides on subterranean termite colonies over foraging distances: colony elimination versus localized termite exclusion. Journal of Economic Entomology 111: 2317-2328. https://doi.org/10.1093/jee/toy210
Chouvenc T, Scheffrahn RH, Buss L. 2022. Termite Species Distribution in Florida and UF Termite Identification Services: ENY-2079/IN1360, EDIS, 2022. https://doi.org/10.32473/edis-in1360-2022
Eyer PA, Blumenfeld AJ, Johnson LN, Perdereau E, Shults P, Wang S, Dedeine F, Dupont S, Bagnères AG, Vargo EL. 2021. Extensive human‐mediated jump dispersal within and across the native and introduced ranges of the invasive termite Reticulitermes flavipes. Molecular Ecology 30: 3948-3964. https://doi.org/10.1111/mec.16022
Janowiecki MA, Austin JW, Szalanski AL, Vargo EL. 2021. Identification of Reticulitermes subterranean termites (Blattodea: Rhinotermitidae) in the Eastern United States using inter-simple sequence repeats. Journal of Economic Entomology 114: 1242-1248. https://doi.org/10.1093/jee/toab028
Oi F, Davis J, McConnell J, Corbus J, Nelson N, Atkinson M. 2020. Termite Prevention and Control: ENY-2044/IN1277, EDIS. https://doi.org/10.32473/edis-in1277-2020
Rust M, and Su N-Y. 2012. Managing social insects of urban importance. Annual Review of Entomology 57: 355–375. https://doi.org/10.1146/annurev-ento-120710-100634
Scheffrahn RH, Su N-Y. 2021. Asian subterranean termite, Coptotermes gestroi (=havilandi) (Wasmann) (Insecta: Isoptera: Rhinotermitidae): EENY128/IN285, revised 4/2021. EDIS, 2002. https://doi.org/10.32473/edis-in285-2000
Scheffrahn RH, Su N-Y. 2021. West Indian subterranean termite, Heterotermes cardini (Insecta: Isoptera: Rhinotermitidae). EENY282/IN558, revised 3/2021. EDIS, 2000.
Scheffrahn RH, Mangold JR, Su N-Y. 1988. A survey of structure-infesting termites of peninsular Florida. Florida Entomologist 71: 615–630. https://doi.org/10.2307/3495021
Scheffrahn, R.H., Su, N.Y., Cabrera, B. and Kern Jr, W., 2021. Cuban Subterranean Termite (proposed), Florida Dampwood Termite (old unofficial name), Prorhinotermes simplex (Hagen) (Insecta: Isoptera: Rhinotermitidae): EENY282/IN558, Rev. 2021. EDIS, 2004(18). https://doi.org/10.32473/edis-in558-2003
Shults P, Richardson, S, Eyer PA, Chura M, Barreda H, Davis RW, Vargo EL. 2021. Area-wide elimination of subterranean termite colonies using a novaluron bait. Insects 12: 192. https://doi.org/10.3390/insects12030192
Su N-Y. 2005. Response of the Formosan subterranean termites (Isoptera: Rhinotermitidae) to baits or nonrepellent termiticides in extended foraging arenas. Journal of Economic Entomology 98: 2143-2152. https://doi.org/10.1093/jee/98.6.2143
Su N-Y, Scheffrahn RH. 2020. Formosan Subterranean Termite, Coptotermes formosanus Shiraki (Insecta: Isoptera: Rhinotermitidae): EENY121/IN278 rev. 04/2020. EDIS 2002. https://doi.org/10.32473/edis-in278-2000
Su N-Y, Ban PM, Scheffrahn, RH. 1993. Foraging populations and territories of the eastern subterranean termite (Isoptera: Rhinotermitidae) in southeastern Florida. Environmental Entomology 22: 1113–1117. https://doi.org/10.1093/ee/22.5.1113