The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Pine bark beetles are frequent pests of stressed pines, Pinus spp., in the southern United States. The five most common southern pine bark beetle species include three in the genus Ips: the sixspined ips, Ips calligraphus (Germar); the eastern fivespined ips, Ips grandicollis (Eichhoff); and the small southern pine engraver, Ips avulsus (Eichhoff); and two species of Dendroctonus: the southern pine beetle, Dendroctonus frontalis Zimmermann, and the black turpentine beetle, Dendroctonus terebrans (Olivier).
Like other pine bark beetles, Ips pine engravers live predominantly in the inner bark, where they breed and feed on phloem tissue. Pines successfully colonized by Ips engravers, if not already dead, are killed by adult and larval feeding in the phloem (which can girdle the tree) and by colonization of the sapwood with blue-stain fungi that the beetles introduce. The blue-stain fungi spread into the xylem and block water flow, serving to hasten tree mortality (Connor and Wilkinson 1983; Kopper et al. 2004).
Ips beetles usually colonize only those trees that are already stressed, declining, or fallen due to other environmental or biotic factors. Ips also readily colonize cut logs and slash and are attracted to fresh pine odors. Infestations may occur in response to drought, root injury or disease, timber management activities, lightning strikes, or other stresses, and sometimes occur in association with attacks by Dendroctonus frontalis or Dendroctonus terebrans (Anderson and Anderson 1968; Lovelady et al. 1991; Miller 1983). When populations of Ips beetles are sufficiently high, they can overcome the defenses of apparently healthy trees by attacking in large numbers. However, Ips outbreaks are greatly limited in duration and spatial scale compared to outbreaks of the more aggressive D. frontalis (Anderson 1977).
Distribution
All three Ips species can be found throughout Florida in areas where pines occur. Ips calligraphus has two recognized subspecies, Ips calligraphus, found throughout much of the eastern US, north to southern Ontario, Canada, and Ips calligraphus ponderosae, a western US subspecies.
The distributions of both Ips calligraphus and Ips grandicollis north of the southern pines coincide with the range of pitch pine (Pinus rigida Mill), although they will affect any pine species within that range. Ips avulsus is restricted to the southeastern states, from southern Pennsylvania to Florida and Texas (USDA Forest Service 1985).
Description
Adults
Adults are small (approx. 2 to 6 mm (0.08 to 0.24 in) in length), cylindrical, reddish-brown to black, with the head generally concealed by the pronotum when viewed from above. The posterior portion of the elytra (wing covers) is distinctively hollowed-out, coarsely punctured, and bordered with multiple spines.
The three southern species can be distinguished by body length and number of spines along each side of the elytral declivity:
Sixspined ips, Ips calligraphus, 3.5 to 6.5 mm (0.14 to 0.26 in) long, six spines per side.

Credit: David T. Almquist, University of Florida

Credit: David T. Almquist, University of Florida
Eastern fivespined ips, Ips grandicollis, 2.8 to 4.7 mm (0.11 to 0.19 in) long, five spines per side.

Credit: David T. Almquist, University of Florida

Credit: David T. Almquist, University of Florida
Small southern pine engraver, Ips avulsus, 2.3 to 2.8 mm (0.1 in) long, four spines per side.

Credit: David T. Almquist, University of Florida
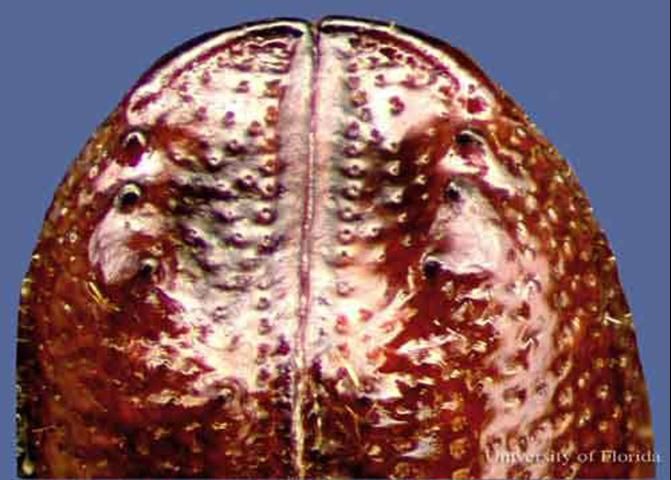
Credit: David T. Almquist, University of Florida
Dorsal comparisons of the elytral apices under magnification may also aid in distinguishing species.
Eggs
Eggs are oblong (ca. 1.0 mm x 0.5 mm (0.19 x 0.02 in)) and pearly white.
Larvae
Larvae are small, whitish, legless, and grub-like with reddish colored heads that are <1 mm (0.04 in) wide.
Pupae
Pupae are waxy-white and similar in size to adults.
(All descriptions from USDA Forest Service 1985, Connor and Wilkinson 1983).
Biology
Adult male Ips beetles are responsible for host selection, principally attacking trees that are stressed, damaged, or recently killed (Coulson and Witter 1984). Males release two primary types of aggregation pheromones, one produced when successfully feeding and the other in response to the presence of defensive resin produced by the tree. These pheromones attract both females and males in numbers that can overwhelm a tree's defense mechanisms. The highest rates of aggregation occur when both pheromone types are produced, indicating that the tree is susceptible to colonization yet still capable of activating its defenses (Vité et al. 1972).
The adult male bores into the phloem and excavates a nuptial chamber, where it mates with one to five (commonly three) females. After mating, each female excavates an egg gallery that extends away from the nuptial chamber and usually parallel to the wood grain, resulting in an overall I-, H- or Y-shaped gallery pattern.

Credit: Ronald F. Billings, Texas Forest Service; http://www.forestryimages.org

Credit: Wayne N. Dixon, Florida DOACS

Credit: Ronald F. Billings, Texas Forest Service; http://www.forestryimages.org
Eggs are deposited in niches along the sides of the egg galleries. Larvae tunnel in the phloem perpendicular to the egg galleries and eventually pupate in individual cells excavated in the inner bark. After pupation, the adult will feed for a short time in the phloem before emerging through the bark, leaving small, scattered emergence holes (USDA Forest Service 1985). Newly-emerged adults can fly as far as four miles in their first dispersal flight to find a new host tree (Kinn 1986).
Development is slower in cool temperatures and the time required to complete the life cycle varies from a few weeks in the summer to several months through the winter. Ips calligraphus and Ips grandicollis can complete their life cycles within 25 days during the summer and can produce eight generations per year in Florida (Dixon 1984), while the Ips avulsus life cycle can take as little as 18 days, producing 10 generations per year. Generations commonly overlap and all life stages may overwinter in the tree (Connor and Wilkinson 1983).
The three species of Ips tend to colonize different parts of the tree, although there is considerable overlap between these territories (Coulson and Witter 1984). Ips calligraphus usually attacks the lower bole or portions of stumps, trunks, and large limbs greater than 10 cm (4 in) in diameter (Connor and Wilkinson 1983). Ips grandicollis prefers to infest recently felled trees and slash, but also can be found infesting weakened living trees, most heavily on large limbs and the mid to upper bole of the host. Ips avulsus prefers small-diameter slash, but will attack groups of young trees and the crowns of large trees (USDA Forest Service 1985). Ips avulsus shows a higher degree of aggregation behavior than some other Ips species (Mason 1970).
Hosts
All three southern Ips species can infest any pine species within their range, and occasionally other conifers such as spruce, hemlock, and fir. Common hosts in Florida include loblolly pine (Pinus taeda L.), longleaf pine (Pinus palustris Mill.), pond pine (Pinus serotina Michx.), sand pine (Pinus clausa (Chapm. ex Engelm.) (Vasey ex Sarg.)), shortleaf pine (Pinus echinata Mill.), slash pine (Pinus elliottii Engelm.), and spruce pine (Pinus glabra Walt.).
Detection
Often the first noticeable indication of an Ips infestation is the fading of foliage from green to yellow to reddish brown as the host tree wilts due to plugging of the xylem by blue-stain fungi.

Credit: John L. Foltz, University of Florida

Credit: John L. Foltz, University of Florida
These color changes can occur in two to four weeks in warm weather but may take several months in the winter. In cooler weather, the beetles have frequently vacated the tree by the time the needles fade. Early signs of attack include the accumulation of reddish-brown boring dust on the bark, nearby cobwebs, or understory foliage.

Credit: Albert E. Mayfield III, Florida FDACS
If there is sufficient resin pressure within the host, attacked trees will exhibit dime-sized, whitish or reddish-brown globs of resin and boring dust called "pitch tubes" on the bark at each point of beetle attack.

Credit: Wayne N. Dixon, Florida FDACS
Unlike those of the southern pine beetle, Ips pitch tubes are more commonly seen on the surface of bark plates than in bark crevices. After beetles emerge from the tree, scattered circular emergence holes (1 to 3 mm (0.04 to 0.12 in) in diameter) can be observed on the outer bark. By removing a section of the outer bark, the characteristic Y-, I-, or H-shaped galleries may be observed in the phloem or engraved on the outer sapwood (Connor and Wilkinson 1983).
These gallery patterns are sometimes obscured by larval galleries of other phloem borers in the families Cerambycidae (roundheaded borers) and Buprestidae (flatheaded borers) that readily colonize dead pines.
Prevention and Management
The strategies for preventing damage and controlling the spread of Ips beetles essentially involve promoting tree vigor and reducing the amount of vulnerable host material within the stand.
Preventative strategies in forest stands include:
- planting species that are appropriate to the site,
- thinning dense, overstocked stands,
- conducting prescribed burns or other treatments to control competing understory vegetation,
- removing and/or salvaging damaged, declining, or recently-dead trees,
- avoiding damage to residual stand when conducting management operations, and
- lopping and scattering or removing logging slash.
(From Connor and Wilkinson 1983; Dixon 1984; Thatcher and Barry 1982)
As for control, when Ips infestations are small and/or sparsely scattered throughout a stand, the best course of action is often to let them die out on their own. Cutting and removal of isolated infested trees or small "spot" infestations with buffer strips (as is done to control Dendroctonus frontalis infestations) is not recommended. Observations in Florida suggest that such selective removals may increase the likelihood of Ips problems by producing fresh host odors, logging slash, and additional stress or injury to the residual stand. If scattered mortality is progressing to unacceptable levels, a stand-level clearcut or a contiguous block removal of a generally infested area may be preferable to selection harvests.
For urban and residential landscape trees, preventative strategies include the following:
- avoiding compaction of, physical damage to, or paving over the root zones of pines,
- providing adequate spacing (15 to 20 feet) between trees,
- minimizing competing vegetation beneath pines,
- maintaining proper soil nutrient and pH status by employing an acidic needle or pine bark mulch over the root zone in place of turf grasses that require frequent irrigation, and
- providing supplemental deep watering during extended drought periods.
In some cases, the application of an approved insecticide that coats the entire tree bole may be warranted to protect high-value landscape trees from infestation; contact your local UF/IFAS Extension office for current insecticide recommendations.
When infested trees are removed, care should be taken to avoid injury to surrounding pines. There is no effective way to save an individual tree once it has been successfully colonized by Ips beetles (Connor and Wilkinson 1983; Dixon 1984; Thatcher et al. 1978).
Selected References
Anderson NH, Anderson DB. 1968. Ips bark beetle attacks and brood development on a lightning-struck pine in relationship to its physical decline. Florida Entomologist 51: 23–30.
Anderson RF. 1948. Host selection by the pine engraver. Journal of Economic Entomology 41: 596–602.
Anderson RF. 1977. Dispersal and attack behavior of the southern pine engraver Ips grandicollis Eichh., Coleoptera, Scolytidae. Pg. 17–23 In Technical Bulletin 310. Technical Bulletin of the Agricultural Experiment Station, University of Minnesota.
Connor MD, Wilkinson RC. 1983. Ips bark beetles in the South. USDA Forest Service, Washington, D.C. Forest Insect & Disease Leaflet 129. 8 p.
Coulson RN, Witter JA. 1984. Forest Entomology: Ecology and Management. John Wiley & Sons, Inc. 315–318.
Dixon WN. 1984. Ips engraver beetles. FDACS, Division of Forestry. Forest and Shade Tree Pests Leaflet No. 2. 2 p.
Kinn DN. 1986. Studies on the flight capabilities of Dendroctonus frontalis and Ips calligraphus: preliminary findings using tethered beetles. USDA Forest Service Research Note SO-324. 3 p.
Kopper BJ, Klepzig KD, Raffa KF. 2004. Components of antagonism and mutualism in Ips pini-fungal interactions: relationship to a life history of colonizing highly stressed and dead trees. Environmental Entomology 33: 28–34.
Lovelady CN, Pulley PE, Coulson RN, Flamm RO. 1991. Relation of lightning to herbivory by the southern pine bark beetle guild (Coleoptera: Scolytidae). Environmental Entomology 20: 1279–1284.
Mason RR. 1970. Comparison of flight aggregation in two species of southern Ips (Coleoptera: Scolytidae). Canadian Entomologist 102: 1036–1041.
Miller MC. 1983. Lightning strike simulation for studying southern pine bark and engraver beetle attacks. USDA Forest Service Research Note SO-296. 4 p.
Thatcher RC, Barry PG. 1982. Southern pine beetle. USDA Forest Service, Washington, D.C. Forest and Disease Leaflet No. 49. 7 p.
Thatcher RC, Coster JE, Payne TL. 1978. Southern pine beetles can kill your ornamental pine. USDA Forest Service, Combined Forest Pest Research Development Program, Washington, D.C. Home and Garden Bulletin 226. 15 p.
USDA Forest Service. 1985. Insects of Eastern Forests. Miscellaneous Publication No. 1426. Washington, D.C. 358–359.
Vité JP, Bakke A, Renwick JAA. 1972. Pheromones in Ips (Coleoptera: Scolytidae): occurrence and production. Canadian Entomologist 104: 1967–1975.