The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids, and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The larvae of the small, uncommon harvester butterfly, Feniseca tarquinius (Fabricius), are the only strictly carnivorous butterfly caterpillars in the United States.
Distribution
Found in swampy areas and woodlands, particularly near water, from southern Canada south to central Florida and central Texas. Highly localized with adults generally remaining in close proximity to woolly aphid hosts.
Description
Adults
The wings are orange on the interior, bordered with black on the dorsal surface and burnt-orange with darker spots edged with white on the ventral surface (Figure 1).

Credit: Donald W. Hall, UF/IFAS
Eggs
The eggs are greenish-white and spherical with faint sculpturing (Figure 2).

Credit: Donald W. Hall, UF/IFAS
Larvae
The larvae (Figures 3–5) are small (to 1.9 cm in length) and slug-like. Full-grown larvae are brightly patterned with gray, yellow and white, and covered with bristly hairs; the pattern is often obscured with the white wax produced by the prey (Minno et al. 2005).

Credit: Jerry F. Butler, UF/IFAS
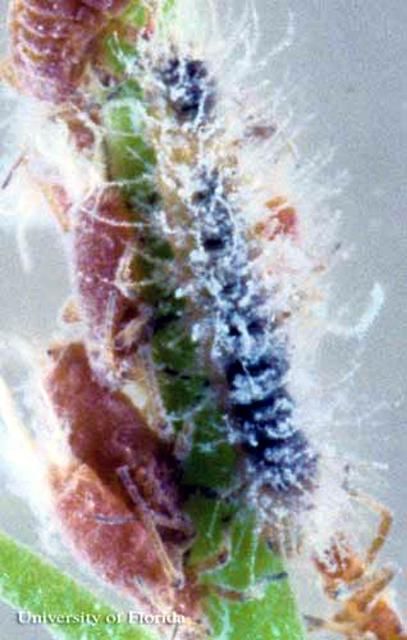
Credit: Jerry F. Butler, UF/IFAS

Credit: Jerry F. Butler, UF/IFAS
Pupae
The pupae are off-white and have a pattern that resembles the face of a lizard or monkey (Krizek 1995) (Figure 6).

Credit: Jerry F. Butler, UF/IFAS
Life Cycle and Biology
There are two to three generations in Canada and the northern US and from three to six generations in the southern US. Eggs are laid singly on leaves or stems near colonies of the woolly aphid prey. Caterpillars are present from June in the North and from February through early November in Florida.
Overwintering is by the pupal (chrysalis) stage (Allen 1997).
Because the harvester caterpillar is carnivorous, development proceeds very rapidly, with the larval stage being completed in as little as eight days. Harvester larvae have only four larval instars. Most other butterflies have five (Layberry et al. 2002). First instar larvae may restrain their larger aphid prey with silk prior to attacking them (D.W. Hall, unpublished observations).
Some harvester caterpillars cover themselves with the remains of woolly aphids they have eaten. The carcasses are tied on with silk, perhaps to protect the caterpillars from predacious ants (that tend and protect the aphids) and other natural enemies. Harvester caterpillars are less likely to conceal themselves when their woolly aphid prey is tended by Camponotus and Formica ants (Youngsteadt and Devries 2005).
Lohman et al. (2006) reported that the caterpillars share part of the cuticular hydrocarbon profile of the aphids and may be protected from the aphid-attending ants and protected by the ants from other predators by this chemical mimicry. Although harvester larvae lack the secretory and call-production organs of other ant-attended lycaenids (Youngsteadt and Devries 2005), they are sometimes attended by ants (Wagner 2005). Interestingly, harvester pupae do have well-developed stridulatory organs (Douglas 1986). The function of these organs in the pupae is not known.
The proboscis of harvester adults is very short, and they do not feed on floral nectar. Instead, they feed on aphid honeydew, dung, sap, and also sip from mud (Scott 1986). Because the adults are small in size, spend most of their time in the locality of their aphid prey, have an erratic flight, and do not feed at flowers, they are not commonly seen. Therefore, they are probably perceived as being more uncommon than they actually are (Wagner 2005).
Hosts
Harvester larvae are predacious on woolly aphids of at least five genera: Meliarhizophagus, Neoprociphilus, Pemphigus, Prociphilus, and Schizoneura (Iftner et al. 1992, Minno et al. 2005, Scott 1986, Opler and Krizek 1984); and possibly on other Homoptera.
The common prey species in Florida are woolly maple aphids, Neoprociphilus aceris (Monell) (Figure 7), that suck sap from earleaf greenbriar (Smilax auriculata Walter), saw greenbriar (Smilax bona-nox L.), cat greenbrier (Smilax glauca Walter), and bristly greenbrier (Smilax tamnoides L.), in the smilax family (Smilacaceae); as well as woolly alder aphids, Prociphilus tesselatus (Fitch) (Figure 8) (formerly Paraprociphilus tesselatus Fitch) that feed on hazel alder (Alnus serrulata (Aiton)Willd.), in the birch family (Betulaceae) (Minno et al. 2005).

Credit: Donald W. Hall, UF/IFAS

Credit: Donald W. Hall, UF/IFAS
Selected References
Allen TJ. 1997. The Butterflies of West Virginia and their Caterpillars. University of Pittsburgh Press. Pittsburgh, Pennsylvania. 388 pp.
Douglas MM. 1986. The Lives of Butterflies. The University of Michigan Press. Ann Arbor, Michigan. 241 pp.
Iftner DC, Shuey JA, Calhoun JV. 1992. Butterflies and Skippers of Ohio. Ohio Biological Survey Bulletin. New Series Bol. 9 No. 1. College of Biological Sciences. The Ohio State University. Columbus, Ohio. 212 pp.
Krizek GO. 1995. Butterfly pupae mimicking mammalian (or vertebrate) faces. Holarctic Lepidoptera 2: 74.
Layberry RA, Hall PW, Lafontaine JD. 1998 The Butterflies of Canada. University of Toronto Press. 376 pp.
Lohman DJ, Liao Q, Pierce NE. 2006 Convergence of chemical mimicry in a guild of aphid predators. Ecological Entomology 31: 41-51.
Minno MC, Butler JF, Hall DW. 2005. Florida Butterfly Caterpillars and their Host Plants. University Press of Florida. Gainesville, Florida. 341 pp.
Opler PA, Krizek GO. 1984. Butterflies East of the Great Plains. The Johns Hopkins University Press. Baltimore, Maryland. 294 pp.
Scott JA. 1986. The Butterflies of North America: A Natural History and Field Guide. Stanford University Press. Stanford, California. 583 pp.
Wagner DL. 2005. Caterpillars of Eastern North America. Princeton University Press. Princeton, New Jersey. 512 pp.
Youngsteadt E, Devries PJ. 2005. The effects of ants on the entomophagous butterfly caterpillar Feniseca tarquinius, and the putative role of chemical camouflage in the Feniseca-Ant interaction. Journal of Chemical Ecology 31: 2091–2109.