Introduction
The horn fly, Haematobia irritans irritans (Linnaeus), is one of the most economically important pests of cattle worldwide. It is an obligate blood-feeding ectoparasite, feeding almost exclusively on cattle. Just in the United States, hundreds of millions of dollars in losses are attributed to the horn fly annually, while additional millions are spent annually on insecticides to reduce horn fly numbers (Kunz et al. 1991, Byford et al. 1992, Cupp et al. 1998).
Synonymy
Conops irritans Linnaeus, 1758
Haematobia cornicola Williston, 1889
Haematobia serrata Robineau-Desvoidy, 1830
Lyperosia meridionalis Bezzi, 1911
Lyperosia rufifrons Bezzi, 1911
Distribution
The horn fly was introduced to North America from France in 1887 (Bruce 1938). This pest is now found throughout the Americas, as well as in Europe, Asia, and the non-tropical regions of Africa.
Description
Adults
The adult horn flies have brownish-gray or black bodies and are shiny, with slightly overlapping wings that are held flat over the abdomen. The body is 3.5 to 5 mm long, or about half the size of the common house fly, Musca domestica Linnaeus. The head has small, brownish-red antennae that point downward. The thorax has two parallel stripes on the dorsal surface, just behind the head. Both male and female horn flies have piercing-sucking mouthparts and feed exclusively on blood.

Credit: Dan Fitzpatrick, University of Florida

Credit: Dan Fitzpatrick, University of Florida
Horn flies differ from another major cattle pest, the stable fly (Stomoxys calcitrans (Linnaeus)), in several ways. Although both flies have a piercing proboscis, horn flies have longer maxillary palpi relative to the proboscis. Horn flies are also smaller (5 mm in length), and have no major patterns on the dorsal (back) side of their abdomen, while stable flies are 7 to 8 mm long and have a "checkerboard" appearance of the top of the abdomen. Horn flies also must lay eggs in undisturbed, fresh manure, whereas stable flies seldom lay eggs in fresh manure, opting rather for manure-straw mixtures, urine-soaked feed and straw, feeding waste sites, grass clipping piles, and round hay bale feeding sites.

Credit: horn fly—Dan Fitzpatrick, stable fly—Jerry Butler, University of Florida
Eggs
Horn fly eggs are tan, yellow or white when first laid, and then darken to a reddish-brown color prior to hatching. Eggs are oval and concave on one side and convex on the other, and are approximately 1.2 mm long

Credit: Dan Fitzpatrick, University of Florida
Larvae
The newly hatched maggots are white and about 1.5 mm long with a slender pointed head. The spiracles, or openings for breathing, appear as black indentations at the end of the abdomen.
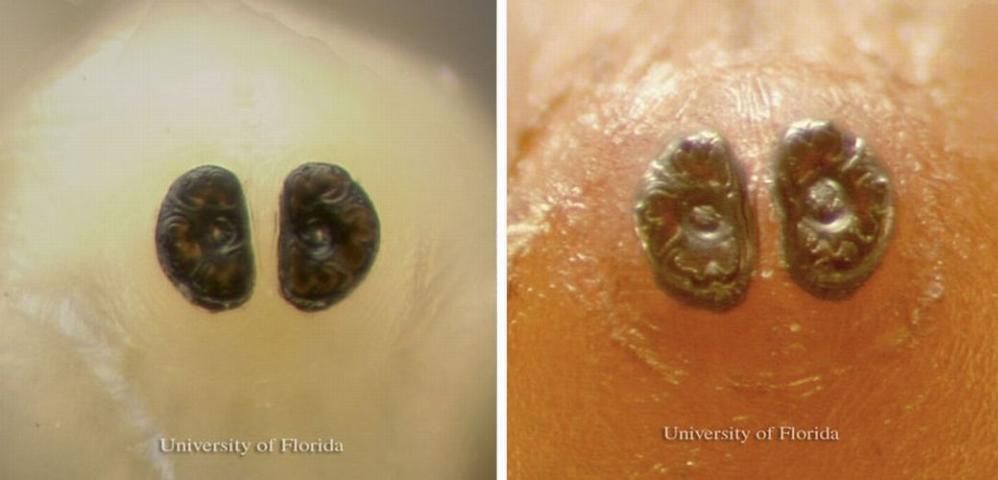
Credit: Dan Fitzpatrick, University of Florida
Pupae
The pupae are 3 to 4 mm long and white at first, the outer pupal covering sclerotizes, or hardens, turning dark reddish-brown over several hours.

Credit: Dan Fitzpatrick, University of Florida
Life Cycle
Cattle manure is the requisite habitat for larval development, and adults principally feed on cattle, with females leaving their host only long enough to lay eggs in fresh manure. The eggs hatch between one to two days after being laid (Foil and Hogsette 1994). Feeding on the fresh dung, larvae develop through three instars in four to eight days before reaching a mature size of 6.5 to 7.5 mm (Lysyk 1991, 1992). Pupation normally requires six to eight days for full maturation (Foil and Hogsette 1994). The time required to complete the life cycle of a horn fly is between 10 and 20 days, depending on the temperature and time of year (Campbell 2006).
When the adult emerges from the pupal case, it takes approximately three days to complete maturation of the reproductive organs that allow for egg production. The adult flies begin mating three to five days following emergence, and adult females start laying eggs three to eight days after emergence. A female horn fly oviposits, or lays, an average of 78 eggs during her adult lifespan of approximately six to seven days, but can lay up to 100-200 eggs (Krafsur and Ernst 1986). Male and female horn flies feed only on blood during their adult stage, whereas other blood-feeding flies, such as the stable fly, will consume nectar.
Though horn flies typically diapause, or hibernate, as pupae over the winter in most subtropical and temperate areas (Mendes and Linhares 1999), horn fly populations are a year-round nuisance to cattle in the southeastern United States, with comparatively lower populations in the winter (Koehler et al. 2005). Fly populations peak in early summer, then decline as the weather becomes hot and dry. In the autumn, populations typically increase again as temperatures drop and rainfall increases, falling off once again after September or October, as late autumn and early winter temperatures set in (Baldwin et al. 2005).
Hosts
Horn flies received this name because of their habit of clustering around the horns of cattle, although they typically prefer to settle on the backs of cattle during the cooler parts of the day and on the belly during the hotter part of the day. They have been known to feed on horses, dogs, swine, and sometimes humans. However, they have a well-documented close association with cattle and typically remain on or near cattle throughout their entire life cycle.
Economic Importance
The horn fly is considered one of the most economically devastating pests of the beef cattle industry in the United States (Byford et al. 1992). It causes annual losses of between US$700 million and $1 billion, while an additional US$60 million is spent annually on insecticides to control infestation (Kunz et al. 1991, Byford et al. 1992, Cupp et al. 1998).
Because of horn fly feeding behavior and the sheer numbers of flies present on the animals, cattle expend a great degree of energy in defensive behavior. This results in elevated heart and respiratory rates, reduced grazing time, decreased feeding efficiency and reduced milk production in cows, which can result in decreased weaning weights (Byford et al. 1992). Extensive horn fly feeding can also severely damage cattle hides, which results in poorer quality leather (Pruett et al. 2003).
Horn flies are commonly reported on beef cattle in large numbers, with thousands of flies occurring on individual animals. Although the average meal size is only 1.5 mg, or 10 µL, of blood per feeding (Kuramochi and Nishijima 1980), each fly takes between 24 to 38 blood meals per day (Foil and Hogsette 1994). Therefore, the sheer numbers of flies infesting an animal, as well as the numbers of blood meals taken daily by each fly, can result in substantial blood loss (Harris et al. 1974).

Credit: Lane Foil, Louisiana State University
The horn fly is also a vector of several pathogens. A filarial nematode, Stephanofilaria stilesi Chitwood, causes stephanofilariasis, a dermatitis characterized by areas of crusted skin on the underside of cattle. Typically found on cattle of the western and southwestern United States and Canada, S. stilesi can affect up to 80 to 90% of a herd (Hibler 1966). However, production losses associated to this nematode or other adverse reactions in cattle have not been reported.
Horn flies also are able to vector several Staphylococcus spp. bacteria, which cause mastitis, or infection of the teats in dairy cows, particularly in summer months (Owens et al. 1998, Gillespie et al. 1999). In addition to the teat damage they cause, feeding flies can introduce the bacteria into open wounds, causing significant infection (Edwards et al. 2000). Cattle producers can reduce cases of mastitis by managing horn fly numbers (Nickerson et al. 1995, Edwards et al. 2000).
Management
Static thresholds have been established based on the numbers of horn flies per animal in order to determine whether the implementation of fly management is economically necessary. Calves and dairy cattle cannot sustain high numbers of flies without sustaining measurable damage; 50+ flies per lactating dairy cow is considered to be of economic importance. Beef cows can tolerate upwards of 200 flies per animal, while bulls can tolerate the greatest number of horn flies (Schreiber et al. 1987, Hogsette et al. 1991).
Chemical control. Insecticide-impregnated ear tags became a popular and effective method for managing horn fly populations, due to the advent of low cost, highly persistent pyrethroid and organophosphate pesticides (Szalanski et al. 1991). In herds affected by horn flies, heifers with ear tags gained up to 50% more weight per day than did untagged control heifers (Sanson et al. 2003). More recently, insecticides formulated into pour-ons are increasingly used. Though insecticide technology has been largely, if not exclusively, relied upon for managing horn flies, resistance to many of the insecticides has been widely reported and demonstrated to occur through several known mechanisms, including target site insensitivity and thorough metabolic detoxification of insecticides (Szalanski et al. 1991). Therefore, use of an integrated pest management approach that utilizes several methods in tandem will allow cattle producers to more effectively reduce adult and larval horn fly populations. A rotation of chemicals with different active ingredients and different application techniques is considered the best approach to managing this fly.
The use of backrubbers and dustbags, which physically apply insecticides to cattle when they brush up against them, can aid control efforts when they are placed in locations where the cattle are forced to brush against them. When insecticide is reapplied to the backrubbers and dustbugs every two to three weeks, they are reasonably effective for managing horn flies (Baldwin et al. 2005).
Feed-through applications, where certain pesticides are mixed into cattle feed, result in the chemical passing through the cattle's digestive tract and hence into the manure. Endectocides also have gained popularity with cattle farmers in recent years under a variety of trade names. These pesticides are injected or topically applied to and absorbed by cattle and are excreted unaltered in the manure. The pesticide remains in the dung and can significantly reduce immature horn fly numbers for up to two months after application (Miller et al. 1981, Lysyk and Colwell 1996, Floate et al. 2001). Another approach to this technique, the bolus, provides several weeks worth of control from a single treatment. Boluses are essentially long-lasting pills that are deposited into the animal's stomach, where they slowly release the insecticide into the manure. Both of these techniques kill only the immature stages of the horn fly and do not affect the adult flies feeding on the animals. Therefore, because the adult flies are not killed, and because new adult flies may emigrate from nearby untreated herds, feed-throughs are not considered cure-all treatments (Baldwin et al. 2005).
Biological insecticides also have gained popularity as alternatives to pyrethroid or organophosphate pesticides. Bacillus thurigiensis Berliner (Bt), a well-known bacterium used as a biological insecticide, is effective against a range of insect pests. Although there are no products for horn fly control on the market containing Bt, recent research has indicated that several strains of Bt are highly toxic to horn fly larvae (Lysyk et al. 2010).
Florida Insect Management Guide for horn flies
Mechanical Control
An old, and perhaps effective, non-chemical control tactic that has been critically evaluated in recent years is the walk-through horn fly trap. These traps utilize the horn fly's reluctance to enter a darkened building to remove the flies from the animals and then trap or kill the flies with sticky traps or electrocution as they leave the animals. More modern designs of this technique are reported to provide up to an 85% reduction of fly numbers (Watson et al. 2002).

Credit: Phillip Kaufman, University of Florida
Biological Control
A number of natural predators, parasitoids, and competitors have been examined as agents for suppression of horn fly numbers. Dung beetles of the family Scarabaeidae, as well as other predaceous beetles of the families Staphylinidae and Histeridae, are important natural predators of larval horn flies in the manure (Hu and Frank 1996, Oyarzún et al. 2008). Interestingly, the red imported fire ant, Solenopsis invicta Buren, also reduces immature horn fly numbers in cattle dung pats as well as through predator activity (Summerlin et al. 1984), but may cause additional problems by killing the other predators and by stinging the cattle, particularly calves (Hu and Frank 1996).

Credit: Phillip Kaufman, University of Florida
Parasitoid wasps of the families Pteromalidae and Chalcididae, which are not pests of people but naturally attack horn flies, have been assessed as potential control agents for use against horn flies in the United States (Geden et al. 2006). These wasps, including Spalangia and Muscidifurax spp., lay their eggs in fly pupae, and the wasps' offspring feed internally on the fly and eventually kill it. To date, horn fly control has not been accomplished solely using naturally-occurring or augmentative biological control principally due to the widely distributed cattle dung pats (and therefore horn fly pupae) and difficulty in getting released wasps to these sites. Cattle producers are encouraged to protect these natural enemies of the horn fly, as without them populations would assuredly be much higher.

Credit: Jerry Butler, University of Florida
Selected References
Baldwin JL, Foil LD, Hogsette JA. (May 2005). Important fly pests of Louisiana beef cattle. LSUAgCenter. http://www.lsuagcenter.com/NR/rdonlyres/AF963495-28CE-4190-BB5D-C675C27BFA78/8364/pub2617flypests4.pdf (28 March 2011).
Bruce WG. 1938. A practical trap for the control of horn flies on cattle. Journal of the Kansas Entomological Society 11: 88–93.
Byford RL, Craig ME, Crosby BL. 1992. A review of ectoparasites and their effect on cattle production. Journal of Animal Science 70: 597–602.
Campbell JB. (June 2006). Horn fly control on cattle. University of Nebraska-Lincoln Extension Publication. http://www.ianrpubs.unl.edu/epublic/live/g1180/build/g1180.pdf (28 March 2011).
Cupp EW, Cupp MS, Ribeiro JMC, Kunz SE. 1998. Bloodfeeding strategy of Haematobia irritans (Diptera: Muscidae). Journal of Medical Entomology 35: 591–595.
Edwards JF, Wikse SE, Field RW, Hoelscher CC, Herd DB. 2000. Bovine teat atresia associated with horn fly (Haematobia irritans irritans (L))-induced dermatitis, Veterinary Pathology 37: 360–364.
Floate KD, Spooner RW, Colwell DD. 2001. Larvicidal activity of endectocides against pest flies in the dung of treated cattle. Medical and Veterinary Entomology 15: 117–120.
Foil LD, Hogsette JA. 1994. Biology and control of tabanids, stable flies and horn flies. Revue Scientifique et Technique 13: 1125–1158.
Geden CJ, Moon RD, Butler JF. 2006. Host ranges of six solitary filth fly parasitoids (Hymenoptera: Pteromalidae, Chalcididae) from Florida, Eurasia, Morocco, and Brazil. Environmental Entomology 35: 405–412.
Gillespie BE, Owens WE, Nickerson SC, Oliver SP. 1999. Deoxyribonucleic acid fingerprinting of Staphylococcus aureus from heifer mammary secretions and from horn flies. Journal of Dairy Science 82: 1581–1585.
Harris RL, Miller JA, Frazar ED. 1974. Horn flies and stable flies: feeding activity. Annals of the Entomological Society of America 67: 891–894.
Haufe WO. 1982. Growth of range cattle protected from horn flies Haematobia irritans by ear tags impregnated with fenvalerate. Canadian Journal of Animal Science 62: 567–573.
Hibler CP. 1966. Development of Stephanofilaria stilesi in horn fly. Journal of Parasitology 52: 890–898.
Hogsette JA, Prichard DL, Ruff JP. 1991. Economic effects of horn fly (Diptera: Muscidae) populations on beef cattle exposed to three pesticide treatment regimes. Journal of Economic Entomology 84: 1270–1274.
Hu GY, Frank JH. 1996. Effect of the red imported fire ant (Hymenoptera: Formicidae) on dung-inhabiting arthropods in Florida. Environmental Entomology 25: 1290–1296.
Kerlin RL, Allingham PG. 1992. Acquired immune response of cattle exposed to buffalo fly (Haematobia irritans exigua). Veterinary Parasitology 43: 115–129.
Koehler, PG, Butler JF, Kaufman PE. (December 2005). Horn flies. EDIS. https://edis.ifas.ufl.edu/ig137 (28 March 2011).
Krafsur ES, Ernst CM. 1986. Phenology of horn fly populations (Diptera: Muscidae) in Iowa, USA. Journal of Medical Entomology 23: 188–195.
Kuramochi K, Nishijima Y. 1980. Measurement of the meal size of the horn fly, Haematobia irritans (L.) (Diptera: Muscidae), by the use of amaranth. Applied Entomological Zoology 15: 262–269.
Lysyk TJ. 1991. Use of life-history parameters to improve a rearing method for horn fly, Haematobia irritans irritans (L) (Diptera, Muscidae), on bovine hosts. Canadian Entomologist 123: 1199–1207.
Lysyk TJ. 1992. Effect of larval rearing temperature and maternal photoperiod on diapause in the horn fly (Diptera, Muscidae). Environmental Entomology 21: 1134–1138.
Lysyk TJ, Colwell DD. 1996. Duration of efficacy of diazinon ear tags and ivermectin pour-on for control of horn fly (Diptera: Muscidae). Journal of Economic Entomology 89: 1513–1520.
Lysyk TJ, Kalischuk-Tymensen LD, Rochon K, Selinger LB. 2010. Activity of Bacillus thuringiensis isolates against immature horn fly and stable fly (Diptera: Muscidae). Journal of Economic Entomology 103: 1019–1029.
Mendes J, Linhares AX. 1999. Diapause, pupation sites and parasitism of the horn fly, Haematobia irritans, in south-eastern Brazil. Medical and Veterinary Entomology 13: 180–185.
Miller JA, Kunz SE, Oehler DD, Miller RW. 1981. Larvicidal activity of Merck MK-933, an avermectin, against the horn fly, stable fly, face fly, and house fly. Journal of Economic Entomology 74: 608–611.
Nickerson SC, Owens WE, Boddie RL. 1995. Mastitis in dairy heifers - initial studies on prevalence and control, Journal of Dairy Science 78: 1607–1618.
Owens WE, Oliver SP, Gillespie BE, Ray CH, Nickerson SC. 1998. Role of horn flies (Haematobia irritans) in Staphylococcus aureus-induced mastitis in dairy heifers. American Journal of Veterinary Research 59: 1122–1124.
Oyarzún, MP, Quiroz A, Birkett MA. 2008. Insecticide resistance in the horn fly: alternative control strategies. Medical and Veterinary Entomology 22: 188–202.
Pruett JH, Steelman CD, Miller JA, Pound JM, George JE. 2003. Distribution of horn flies on individual cows as a percentage of the total horn fly population. Veterinary Parasitology 116: 251–258.
Sanson DW, DeRosa AA, Oremus GR, Foil LD. 2003. Effect of horn fly and internal parasite control on growth of beef heifers. Veterinary Parasitology 117: 291–300.
Schreiber ET, Campbell JB, Kunz SE, Clanton DC, Hudson DB. 1987. Effects of horn fly (Diptera: Muscidae) control on cows and gastrointestinal worm (Nematode: Trichostrongylidae) treatment for calves on cow and calf weight gains. Journal of Economic Entomology 80: 451–454.
Summerlin JW, Petersen HD, Harris RL. 1984. Red imported fire ant (Hymenoptera: Formicidae): effects on the horn fly (Diptera: Muscidae) and coprophagous scarabs. Environmental Entomology 13: 1405–1410.
Szalanski, AL, Black WC, Broce AB. 1991. Esterase staining activity in pyrethroid-resistant horn flies (Diptera: Muscidae). Journal of the Kansas Entomological Society 68: 303–312.
Watson DW, Stringham SM, Denning SS, Washburn SP, Poore MH, Meier A. 2002. Managing the horn fly (Diptera: Muscidae) using an electric walk-through fly trap. Journal of Economic Entomology 95: 1113–1118.