The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The dark rover ant, Brachymyrmex patagonicus Mayr, is a tiny ant, native to Argentina and Paraguay, that was introduced relatively recently to the United States. It is established in the Gulf States and in some urban areas of Arizona and Nevada. It is a nuisance species because alates (winged adults) and foraging workers invade structures, as well as establish nests in structures. Ants in this genus are commonly known as rover ants.
Distribution
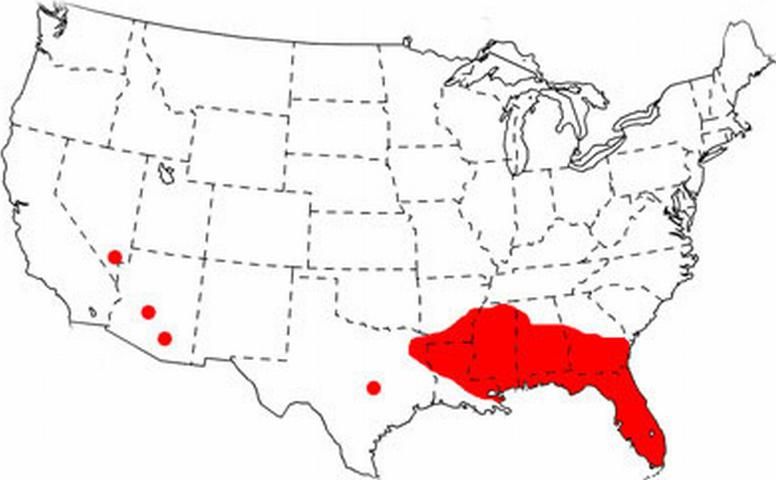
The native range of Brachymyrmex patagonicus Mayr is in Argentina and Paraguay, and it is an introduced species in the United States. It was first observed in the United States in Louisiana in 1976 (Wheeler 1978), but was misidentified at that time as Brachymyrmex musculus Forel (MacGown et al. 2007). Its range has rapidly expanded and it is now well established in Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi and urban centers in the southwest U.S. (Nevada and Arizona). It is more common in northern Florida than in southern Florida, where it may be in direct competition with the closely related, and very abundant, Brachymyrmex obscurior Forel (MacGown et al. 2007). The following map, documenting its distribution, is somewhat out of date, as the species is now established in Houston, Dallas and San Antonio, Texas (Wild 2008), and has subsequently been recorded in South Carolina (MacGown et al. 2010) and southern California (Martinez 2010). The potential range for Brachymyrmex patagonicus may be as far north as Tennessee (MacGown et al. 2010).
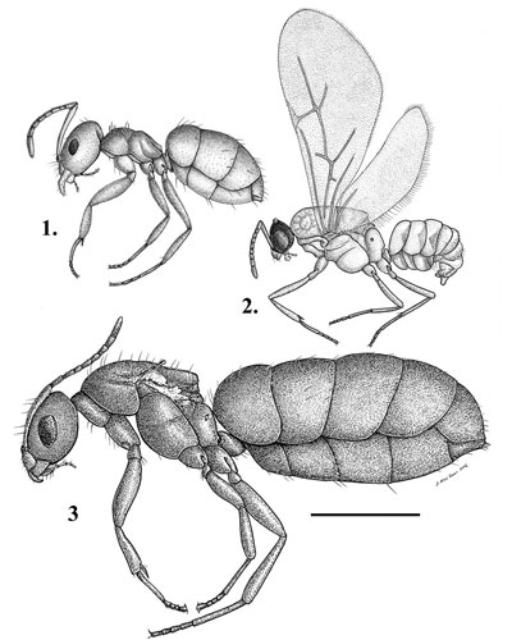
Description
Brachymyrmex patagonicus is a very small brown ant. The body length by caste is: workers 1 to 2 mm, queens 3 mm, males 1 mm.


Credit: Joe A. MacGown, Mississippi Entomological Museum

Credit: Joe A. MacGown, Mississippi Entomological Museum

Credit: Joe A. MacGown, Mississippi Entomological Museum
As with all Brachymyrmex spp., the antennae of Brachymyrmex patagonicus workers have only nine segments (Wild 2008).

Credit: Alex Wild, www.alexanderwild.com
There are several key features that distinguish workers of this species from other members of the genus that are likely to be encountered in the United States (Wild 2008). These features include long hairs on the mesosoma, relatively large eye (compared to other Brachymyrmex spp.), and sparseness of appressed hairs on the dorsal surface of the gaster (Wild 2008).

Credit: Alex Wild, www.alexanderwild.com

Credit: Alex Wild, www.alexanderwild.com
Biology and Behavior
Few studies have been conducted on the biology of this species. An artificial diet that supports reproducing colonies in the laboratory includes 30% honey solution, liquid tuna mixture, and caterpillar pieces (Miguelena and Baker 2010).
Dark rover ants visit extrafloral nectaries for nectar (Miguelena 2011, personal communication; Robbins and Miller 2009; Wild 2008). It is the most common species, compared to three native ant species found on Opuntia stricta cactus in Wakulla County, Florida, on which it shows a spatial preference for parts of the plant with the highest concentration of extrafloral nectaries. Because visitation by ants attracted by cactus extrafloral nectaries has been shown in some cases to provide protection against herbivores, Brachymyrmex patagonicus's potential role in regulating populations of the invasive cactus moth, Cactoblastis cactorum, is under investigation. Interaction with native ant species may be important in this system (Robbins and Miller 2009). This species may also visit hemipterans for honeydew, which may form a major portion of their diet (MacGown et al. 2007).

Credit: Alex Wild, www.alexanderwild.com

Credit: Alex Wild, www.alexanderwild.com
Foraging behavior does not involve trail making, although in the laboratory trails are formed between sub-units of colonies (Miguelena and Baker 2010).
A high level of inter-colony aggression has been observed in the laboratory, and this species is unlikely to be able to establish supercolonies. Persistent multiple queen colonies have been established under laboratory conditions, but polygyny has not yet been observed in the wild. Colonies show a propensity to establish satellite colonies (Miguelena and Baker 2010).
Survival of workers in isolated laboratory colonies is increased when larvae are present. Workers in laboratory satellite colonies without larvae will lay unfertilized eggs that become winged males (Miguelena 2011, personal communication).

Credit: Javier G. Miguelena, University of Arizona
Mating flights may occur year round in Tucson, Arizona. Alates have been observed from May-August in the southeastern states.

Credit: Alex Wild, www.alexanderwild.com

Credit: Alex Wild, www.alexanderwild.com

Credit: Alex Wild, www.alexanderwild.com
Brachymyrmex patagonicus has been known to coexist in close proximity to a wide variety of native and introduced ant species, including some species that are generally intolerant of other ants, including the red imported fire ant, Solenopsis invicta Buren (MacGown et al. 2007), and southern fire ant, Solenopsis xyloni McCook (Miguelena 2011, personal communication).
Habitat
Colonies are formed in soil, at bases of trees, in leaf litter, wood piles, and rubbish heaps. They are found in both natural and disturbed areas, at least in the southeastern states, although there are indications that its occurrence is higher around urban areas and other concentrations of human activity. In landscaped areas, dark rover ants are commonly found in mulch. Nests are also formed within man-made structures (MacGown et al. 2007). In southern California, workers have been found in urban areas foraging on pavement adjacent to turf (Martinez 2010).
Laboratory studies indicate a preference for high levels of moisture (Miguelena 2011, personal communication), and anecdotal reports indicate a tendency to invade bathrooms and kitchens (MacGown et al. 2007). In the arid southwest, it is likely to occur only in limited areas, such as irrigated landscapes, where adequate moisture occurs (Miguelena and Baker 2010).
Economic Importance
Brachymyrmex patagonicus is a nuisance pest in the southeastern United States, Texas, and in Arizona, and is not known to cause damage to structures or landscapes. It is not currently a nuisance problem in southern California (Wilen 2011, personal communication; Greenberg 2011, personal communication), where it was first reported in 2010. It does not bite, sting or transmit disease. It invades structures as foraging workers and swarming alates, and in nest establishment. In the desert, structures may be invaded by foragers during the hot, dry spring and summer months. Nests may also be established indoors at those times. Large numbers of alates in swimming pools is another frequent problem.
Management
Due to the rapid range expansion of this species, it is a relatively new pest problem to numerous pest management professionals and university extension staff. In Mississippi, university staff reported in 2005 and 2006 that inquiries about dark rover ants exceeded those of all other ant species. Pest management professionals throughout the range of dark rover ants in the United States have reported difficulty in achieving effective control (MacGown et al. 2007, Wild 2008). Toxicity studies of various pesticide products are underway (Baker 2011, personal communication). Attraction to honeydew and extrafloral nectaries suggests that baiting may be an effective strategy, but research on this has yet to be conducted.
Preliminary laboratory work indicates that this species requires relatively high moisture (Miguelena and Baker 2010), so in some areas, modification of irrigation and other horticultural practices may provide a method of control of colonies outside buildings. Control of excessive moisture within buildings, and in mulch near buildings, may minimize the chance of invasion.
Because alates are attracted to lights, the selection of light bulb type, or placement of light fixtures may affect nest formation in structures.
Selected References
Baker PA. 2011. Personal Communication. University of Arizona, Department of Entomology, Tucson, Arizona.
Greenberg L. 2011. Personal communication. University of California Riverside, Department of Entomology, Riverside, California.
MacGown JA. (February 2011). Brachymyrmex patagonicus Mayr. Mississippi Entomological Museum. https://mississippientomologicalmuseum.org.msstate.edu/Researchtaxapages/Formicidaepages/genericpages/Brachymyrmex.patagonicus.htm (21 July 2011).
MacGown JA, Hill JG, Deyrup MA. 2007. Brachymyrmex patagonicus (Hymenoptera: Formicidae), an emerging pest species in the southeastern United States. Florida Entomologist 90: 457–464.
MacGown JA, Hill JG, Brown RL. 2010. Dispersal of the exotic Brachymyrmex patagonicus (Hymenoptera: Formicidae) in the United States. Proceedings: Imported Fire Ant Conference, Charleston, South Carolina, March 24–26, 2008: 80–86.
Martinez MJ. 2010. Brachymyrmex patagonicus Mayr southern California specimen records. www.antweb.org. AntWeb. https://www.antweb.org/description.do?genus=brachymyrmex&species=patagonicus&rank=species
Miguelena JG. 2011. Personal communication. University of Arizona, Department of Entomology, Tucson, Arizona.
Miguelena JG, Baker PB. 2010. Why are rover ants (Brachymyrmex patagonicus) so difficult to control? Graduate Student Poster Session, Entomological Society of America Annual Meeting, San Diego, California, December 12–15, 2010.
Robbins M, Miller TEX. 2009. Patterns of ant activity on Opuntia stricta (Cactaceae), a native host-plant of the invasive cactus moth, Cactoblastis cactorum (Lepidoptera: Pyralidae). Florida Entomologist 92: 391–393.
Wheeler GC, Wheeler J. 1978. Brachymyrmex musculus, a new ant in the United States. Entomological News 89: 189-190.
Wild AL. 2008. Myrmecos Blog: Rover Ants (Brachymyrmex patagonicus), an emerging pest species. Myrmecos Blog. https://myrmecos.wordpress.com/2008/05/27/rover-ants-brachymyrmex-patagonicus-an-emerging-pest-species (21 July 2011).
Wilen CA. 2011. Personal communication. University of California Cooperative Extension. Los Angeles, Orange and San Diego counties, California.