This fact sheet is one of a series titled Reclaimed Water Use in the Landscape addressing various issues related to use and management of reclaimed water in urban landscapes.
Introduction
As much as half of the potable water (drinking water) consumed in many Florida communities is used for landscape irrigation. Much of this potable water use can be replaced by reclaimed water, which is treated domestic wastewater that is considered a high quality alternative to potable water for landscape irrigation. Reclaimed water has been safely used in Florida for more than 40 years to irrigate lawns, public green spaces, and crops (primarily citrus). Not only does reclaimed water reduce stress on potable water supplies, but this water source also costs less than potable water and is therefore an appealing choice for irrigation. However, the quality of reclaimed water does differ from potable water, so special management considerations should always be considered when reclaimed water is used in the landscape.
This publication describes three areas of possible concern associated with the use of reclaimed water for landscape irrigation:
-
Soil salinity—the buildup of salts in the soil.
-
Sodicity—the buildup of sodium ions in the soil.
-
Specific ions—ions such as chloride, sodium, and boron that may be toxic to plants.
Each area of concern is discussed below, along with a discussion of how to diagnose potential problems and options for proper irrigation management. The objective of this publication is to inform UF/IFAS Extension agents, state agency personnel, landscape managers, and citizens of ways to manage salts and other ions in irrigated landscapes where reclaimed water is used. Information about the various dissolved constituents in reclaimed water and their behavior in the soil can be found in https://edis.ifas.ufl.edu/ss542 and https://edis.ifas.ufl.edu/ss543.
Soil Salinity
Issue of Concern
Salinity refers to the concentration of salts present in irrigation water or in the soil solution (liquid phase in the soil). Dissolved ions that may be found in irrigation water include the following:
-
Calcium
-
Magnesium
-
Sodium
-
Potassium
-
Bicarbonate
-
Carbonate
-
Chloride
-
Nitrate
-
Boron
-
Sulfate
Nitrate, potassium, calcium, and magnesium are also nutrient sources for plants, while all ions contribute to salt load in the reclaimed water.
The concentration of salts present in reclaimed water used for irrigation is important because salts remain behind and can build up in soil after plants extract the water. The buildup of salts in the root zone of plants may cause discoloration of turf, damage to roots and shoots, and reduced survival of seedlings. Elevated salt concentration in soil can also cause physiological drought, a condition that occurs when salts draw water away from plant roots, thereby inhibiting water uptake and contributing to plant water stress. Drought stress symptoms may occur even when the soil appears to be moist.
Almost all water, even natural rainfall, contains some dissolved salts. Reclaimed water typically has an elevated salt concentration since it is derived from domestic wastewater. In fact, the first concern when examining reclaimed water quality is to evaluate its salinity hazard, which refers to the concentration of total soluble salts present (US EPA, 2004).
Diagnosing a Salinity Problem
The best way to diagnose a potential salinity problem is to determine the salt concentrations in your irrigation water and soil. You can directly determine the salinity of irrigation water with a water quality test. The lab that conducts the test will report salinity as the electrical conductivity of the water (ECw). Pure water is a poor conductor of electricity, but as the water's salt concentration increases, so does its ability to conduct electricity. Electrical conductivity is expressed in units of deci-seimens per meter (dS/m) or milli-mhos per centimeter (mmhos/cm). Water with an ECw value below 0.78 dS/m has few or no detrimental effects on plants and soil, depending on salt sensitivity of the plant species. Once the ECw value of irrigation water reaches 1.56 dS/m or higher, salinity problems may become evident. Table 1 provides interpretation and options for management of high salinity irrigation water.
A soil test can evaluate the extent to which salts have accumulated in irrigated soil. Soil salinity level is likewise reported as an EC value. Table 2 shows how soil EC values relate to plant salinity tolerance.
Salinity problems can be diagnosed by visual inspection of the soil and landscape. Signs of a salinity problem include the following:
-
Plants that show water stress (wilting) even when soil moisture is adequate
-
Turf discoloration that does not respond to nutrient inputs
-
A white surface crust on the soil—a surface that accumulates as salts build up after water is taken up by plants or evaporates (see Figure 1)

Credit: Duncan et al. (2009)
Options for Managing Soil Salinity
Salt is not usually a problem at sites where reclaimed water is used for irrigation (because Florida has sandy soils and high rainfall), though some coastal area soils may have a higher than normal salt concentration. If your reclaimed water is high in salts and you have determined that it is detrimentally affecting plant growth, you may have to switch to an alternative water source or blend potable water with reclaimed water. Alternatively, you can check Table 1 to see what kind of plant tolerance you need and then consult https://edis.ifas.ufl.edu/ep012 that describes relative salt tolerance for a variety of Florida landscape plants. Even if your reclaimed water cannot be used on some salt-sensitive plants like azalea, this water source can still be used for lawns without problems as turfgrasses have a high salt tolerance.
You can also treat the soil that has excessive salts by:
-
Leaching the salts by improving soil drainage—apply more water than is necessary for plant growth to encourage the downward movement of water through the soil. This action will wash away excess salts from the plant root zone. For sandy Florida soils, 2 to 3 inches of water should provide enough leaching to remove 90% of the salts from the upper 1.5 to 2 feet of the soil. Consult https://edis.ifas.ufl.edu/ae091 to calculate necessary leaching fractions.
-
Reducing evaporation—apply mulch to the soil to reduce the evaporation rate. This action will allow more water infiltration through the soil, thus more leaching will take place, taking salts away from the plant root zone.
Sodicity
Issue of Concern
Sodicity is an excess of sodium in soil. Reclaimed water used for irrigation may contain sodium, especially where sodium chloride-based water softeners are used to reduce hard water problems, or in coastal areas where seawater (which is rich in sodium) intrusion is a problem. Sodium applied with reclaimed water becomes a problem if the sodium accumulates in the soil. As sodium concentration increases, soil structure is diminished because sodium causes soil particles to disperse and soil aggregates to break apart. With diminished soil structure, water infiltration rates decline and plant roots receive less water (See Figure 2). Though not always the case, soils impacted by high sodium concentration may also have high concentration of other salts. These soils are classified as saline-sodic soils, and they display the same problems associated with saline soils as described above.
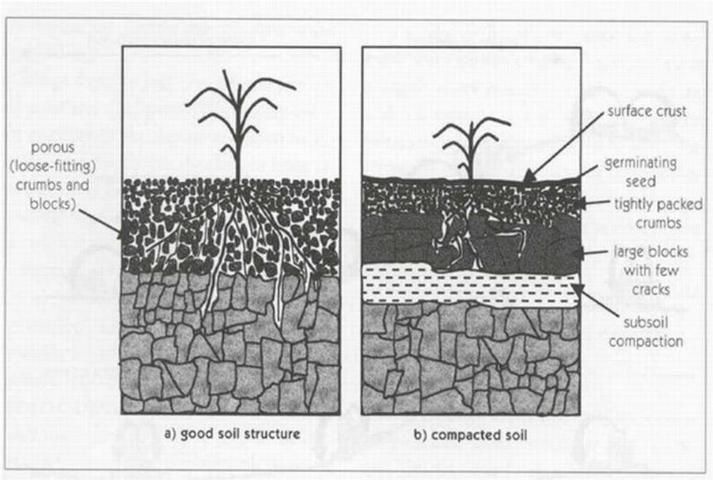
The extent to which sodium can create problems for water infiltration in soil is often called the sodium permeability hazard. The sodium permeability hazard is highest when sodium concentration is high in relation to calcium and magnesium. If calcium and magnesium are present in high enough concentrations, the negative effect of sodium can be offset. Calcium and magnesium have more powerful electrical attraction to soil particles and will displace sodium from soil sites. Likewise, the sodium permeability hazard will be high when concentrations of bicarbonates and carbonates in reclaimed water exceed 120 and 15 mg/L, respectively. This effect occurs because bicarbonates and carbonates combine with calcium and magnesium, taking these out of the soil solution while leaving sodium to accumulate in the soil.
Diagnosing Sodicity Problems
Table 3 shows how reclaimed water can be assessed for its potential to cause sodium-induced water permeability problems by determining one of the following in the water:
-
Sodium adsorption ratio (SAR), which relates sodium concentration to calcium plus magnesium concentration. This ratio assesses how well calcium and magnesium are available to offset sodium.
-
Residual sodium carbonate (RSC), which compares concentrations of bicarbonates and carbonates to calcium and magnesium. RSC reflects the availability of calcium and magnesium to counteract sodium.
You can diagnose a sodicity problem at a site irrigated with reclaimed water by making the following observations:
-
Reduced drainage; water ponds on the soil surface.
-
Reduced seedling emergence and plant rooting problems—because of diminished soil structure and sodium toxicity (see Figure 2).
-
A brownish-black surface crust—due to the dispersion of soil organic matter as soil structure diminishes.
-
A soil that is sticky when wet but forms hard crust upon drying—may not be so much a problem for Florida's sandy soils but can be seen in soils with higher clay contents such as those in Texas.
Managing Sodicity
The use of high-sodium reclaimed water for irrigation can cause a sodicity problem in soil with time. For sandy soils, a reclaimed water SAR value less than 18 milli-equivalents per liter (meq/L) will not generally cause a problem. For a more clayey soil, an SAR value not less than 10 meq/L is desirable (see Table 3). If you suspect a sodium problem, you should contact your reclaimed water provider to obtain information on sodium concentration. You can also have your reclaimed water tested by a lab to determine sodium, but note that concentration of sodium and other ions shows high variability and a one-time test of water may not be representative. If your reclaimed water's SAR value is too high, it is your best option to switch to an alternative water source or blend with high-quality water for irrigation. You can alternatively treat a soil sodicity problem by adding a soluble form of calcium (like gypsum) to the soil. The calcium in the gypsum will exchange with sodium present on soil surfaces, putting the sodium into the soil solution and allowing it to leach.
Correcting 1 acre of sodic soil to a depth of 1 foot requires approximately 1.7 tons of pure gypsum (CaSO4-2H2O) for each milli-equivalent of exchangeable sodium present per 100 grams of soil. A soil test can provide information about how much exchangeable sodium is present in your soil and will provide recommendations for a gypsum amendment. Once you apply gypsum to the soil, the sodium that has been replaced by calcium can then be leached through the soil with extra water. Whereas total soluble salts can be readily flushed from most soils, accumulated sodium may take 1 or more years of leaching before a sodicity problem is corrected. The proactive prevention of a sodic condition is much less expensive and less time-consuming than treating a sodic soil, so determine if there is a sodium hazard associated with your reclaimed water. It is advisable to contact your reclaimed water provider if you suspect sodicity problems in your landscape as they will have more information about the chemical composition and variability in reclaimed water.
Note that if you have problems with both salinity and sodicity, you must treat the sodicity problem first. Otherwise, the soil will not have adequate drainage to support a leaching regimen.
Specific Ions
Issue of Concern
Table 4 summarizes specific ions of concern (chloride, sodium, boron) that may be found in reclaimed water used for irrigation. If present in excess concentration, each of the ions shown can impact plants by root uptake or by direct contact with foliage after overhead sprinkler application, possibly leading to toxicity.
Diagnosing Problems from Specific Ions
Problems with chloride toxicity can be diagnosed by leaf firing (a yellow to tan color on leaf tips) that occurs when excess chloride is transported to the growing shoots of plants (See Figure 3). For most users, simply being aware of the chloride concentration in reclaimed water will help diagnose most problems. Consult Table 4 for recommended concentrations and note that foliar damage does not usually occur until the chloride concentration exceeds 100 mg/L (ppm).

Credit: Duncan et al. (2009)
Boron toxicity in plants is exhibited by yellowing followed by a dark necrosis on the margins of older leaves. Likewise, sodium toxicity can cause discoloration of foliage and leaf burn. Sodium will also compete with calcium in root tissues, causing calcium deficiency and rooting problems in young plants. A comparison of your reclaimed water constituents with Table 4 will enable you to assess the extent to which boron and sodium toxicities are likely to occur. Note that reclaimed water is highly variable due to differences in daily sources of constituents. It is best to contact your reclaimed water provider to obtain information on the chemical constituents present in the reclaimed water. Also, consult Toor and Lusk (2011) Constituents of Concern in Reclaimed Water at https://edis.ifas.ufl.edu/ss543 for pictures and information of specific ions' effect on plants.
Management Options for Chloride, Boron, and Sodium Toxicities
If your reclaimed water contains excessive amounts of these ions, blend with high-quality water (such as potable water), or switch to an alternative water source, or, at a minimum, ensure that you have good drainage to support the movement of toxic ions out of the root zone. Another good management option is to avoid the use of overhead sprinkler irrigation so that irrigation water does not directly contact plant surfaces. Boron leaches quickly from sandy soils and is generally not a problem in Florida. If sodium concentration is high, you will usually see problems associated with reduced water infiltration (see Sodicity, above) before you see problems with toxicity. In this case, your best management option is again to switch to an alternative water source or blend with high-quality potable water. You may also treat excess sodium in the soil by applying soluble calcium (such as gypsum) to the soil and then leaching the soil with excess water.
Summary
Reclaimed water used for irrigation may contain salts that contribute to salinity, sodicity, and/or specific ion toxicity problems in the landscape. Salinity problems can develop when irrigation water has an ECw value greater than 1.56 dS/m. Indications of salinity problems include drought stress and foliage discoloration. The management options for salinity include (1) blending reclaimed water with high-quality potable water or (2) using alternative irrigation water. One of these options should be complimented with the implementation of a drainage program to leach excess salts from the root zone.
Sodicity problems occur when there is excess sodium in the soil, and irrigation water is often the source of excess sodium ions. Sodium-affected soils have impaired water infiltration and will not generally support adequate rooting and seedling emergence. Sodic soils must be treated to remove excess sodium. Amendments containing soluble calcium such as gypsum are a good management option to correct sodicity since the calcium in gypsum will exchange with sodium present on soil surfaces.
If potable water is available, blending or switching to an alternative water source for irrigation will be a much less costly means of preventing sodicity problems in the first place. However, in communities that have a dedicated reclaimed water line, blending or alternating water sources may not be an option. In these communities, the best option may be to grow salt-tolerant plants.
Excess concentrations of chlorine, boron, and sodium can also be directly toxic to plants and will especially cause problems with foliage discoloration, leaf drop, and rooting problems. Again, switch to an alternative water source or blend with potable water if these ions are in excess in your reclaimed water. Other management options include implementing a good drainage program and avoiding the use of overhead sprinkler irrigation, which can minimize most salt problems.
References
Duncan, R.R., Carrow, R., and M. T. Huck. 2009. Turfgrass and Landscape Irrigation Water Quality: Assessment and Management. CRC Press, Boca Raton, FL.
US EPA. 2004. Guidelines for Water Reuse. EPA/625/R-04/108. US EPA, Office of Water, Washington, D. C.
Tables
Irrigation water salinity classifications. Adapted from Duncan et al. (2009). ECw is electrical conductivity of water, expressed in units of deci-siemens per meter (dS/m).