This publication is part of a series titled Soil Phosphorus Storage Capacity (SPSC) for Phosphorus Risk Assessment and Management. The series is intended for use by soil scientists, environmental consultants, state agency personnel, Extension faculty, and others who are interested in management practices and policies that minimize the risk of phosphorus loss from soils.
Introduction
The potential of phosphorus (P) from a given P source to have a negative impact on water quality depends on a number of factors, including the solubility of P in the source and the soil characteristics of the P application site. Many states, including Florida, have adopted a P-Index as a risk assessment tool to determine when a P-based management plan (as opposed to an N-based one) is required. One component of the P-Index is the "P source coefficient," a weighting factor that accounts for differences in P solubility among various organic (e.g., different manure types, biosolids, composts) and inorganic P sources. In this publication, we examine solubility of various P sources, giving special attention to manure and inorganic fertilizers, the two most commonly land-applied P sources in the state. The information in this publication will be useful for soil scientists, environmental consultants, state agency personnel, Extension faculty, and others with an interest in the fate of P from soils due to application of organic and inorganic P fertilizers.
What components in manure are responsible for release of Phosphorus?
Phosphorus exists in soils mainly as the phosphate anion in association with cations such as calcium (Ca), magnesium (Mg), iron (Fe) or aluminum (Al). Using a P fractionation technique (Nair et al. 1995), it is possible to identify the amount of P in manure or manure-amended soil that is associated with the groups of metals (Ca/Mg or Fe/Al). Some of the P is associated with Fe and Al, and some is a component of organic matter. Also, a certain amount of P is highly resistant to P release. This very stable P form is referred to as residual P.
When manure or manure-amended soil comes in contact with water (rain, rising water table), some of the P will be released. The Ca/Mg-P in manure is not stable; rather, it continues to release slowly with time. This release of P from manure and manure-amended soils can continue for a long time (Nair et al. 2011; https://edis.ifas.ufl.edu/ss558), perhaps several decades. How easily the P is released from the manure depends on how strongly the P is held by Ca and Mg, which depends on the form of feed supplement that is provided to cattle. For example, Herrera et al. (2010) found that feeding Ca to lactating dairy cows in the form of the more soluble calcium chloride instead of calcium carbonate resulted in less soluble P in the manure. They also found that maintaining a higher Ca:Mg ratio favored stability of manure P with less P release when in contact with water.
Are there any solubility differences among various manure types?
The ability of dairy and beef manure to release P is illustrated in Figure 1. The most easily released P (labile P) is greater for beef manure (24%) compared with dairy manure (5%). But, total P in dairy manure is approximately 8,000 mg kg-1 compared with 6,000 mg kg-1 in the beef manure.
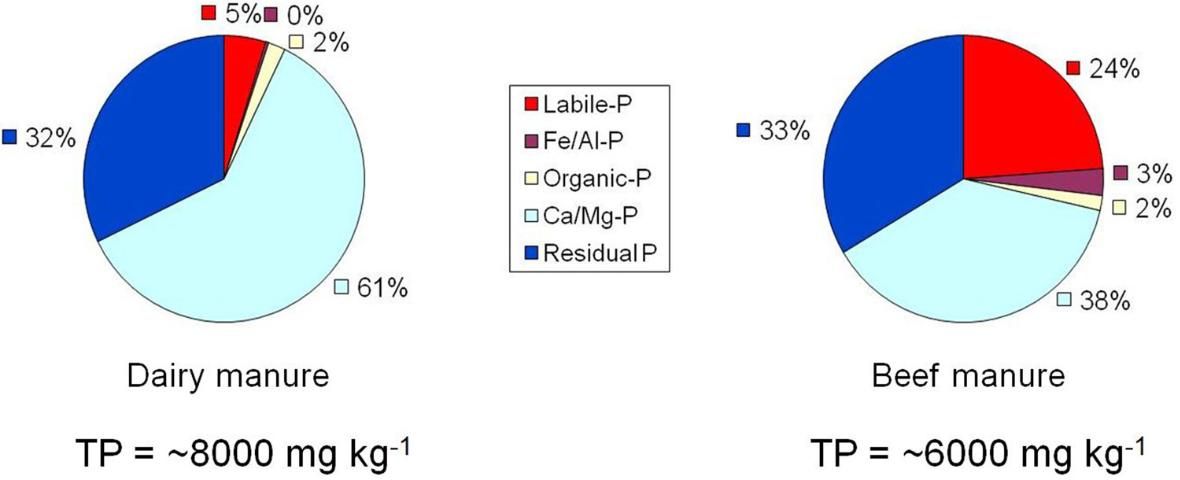
Credits: Nair et al. 2003
The differences in the solubility of dairy and beef manure are likely related to the supplements provided to lactating dairy cows compared with the nature of the Ca/Mg P associated with the forage consumption of free-roaming animals. In this regard, it is likely that the P solubility of beef manure would differ substantially depending on their diet.
How do various P sources in Florida differ in their P solubility?
Some commonly found P sources in Florida differ significantly in P solubility as determined by a one-time extraction of the P source with water (Table 1).
Concentrated superphosphate (CSP), the inorganic P source often used as fertilizer, has the highest P solubility among the various P sources. The two manures and the two biosolid sources also differ in their P solubility, indicating that when land applied at an equal total P rate, the loss of P via various pathways (Nair et al.; https://edis.ifas.ufl.edu/ss558) depends on the P source and not just on the total P content.
How will different P sources affect water quality when the same total P amount is applied?
In a field situation, the movement of P when in contact with water will depend on the solubility of the P source. From Table 1, it appears that the rate of P movement will vary as CSP > Orlando biosolids > poultry manure > dairy manure > compost > Milorganite. This list is not exhaustive, but it illustrates what happens when a given amount of P is applied in different forms. Therefore, P sources labeled as manure, biosolids, or compost can have different impacts on water quality. For example, an inorganic P source (CSP) will likely leave a site faster than manure when applied at the same rate if the amount applied exceeds what is needed for crop uptake. Also, the rate at which P moves through a soil profile will depend on soil components. For example, P will move faster through a soil such as the A and E horizons of Spodosols that have negligible P-retention capacity (Nair et al.; https://edis.ifas.ufl.edu/ss558). However, the P can be held more tightly once it reaches the spodic (Bh) horizon.
What Should We Consider When Applying an Organic or Inorganic Fertilizer
- First, consider the solubility of the P source. The more soluble the P, the more easily it could leave the field.
- Second, consider that the loss of P from the soil will heavily depend on soil components. Therefore, a soil with a large capacity to retain P will be more protective of water quality compared with a soil that releases P easily.
What can be done to help manage P application?
Better management practices can be developed for sustainable agricultural practices by combining knowledge about the risk potential associated with various P sources and the type of soil found at the P application site. In another publication in this series (SL 336/SS541 Understanding Soil Phosphorus Storage Capacity; https://edis.ifas.ufl.edu/ss541), we introduced the soil P storage capacity (SPSC) concept. This concept provides a means of evaluating when a soil will likely release P to the environment.
References
Harris, W. G., V. D. Nair, R. D. Rhue, and D. A. Graetz. 2007. Progress Report for Laboratory-scale Rainfall Simulation. Site-specific Determination of Soil Capacity to Assimilate or Release P Applied as Manure, Fertilizer, Compost, or Biosolids. Report to FDACS. Contract number 0061873. Tallahassee, FL: Florida Department of Agriculture and Consumer Services.
Herrera, D., W. G. Harris, V. D. Nair, M. Josan, and C. R. Staples. 2010. "Effect of Dietary Modifications of Calcium and Magnesium on Reducing Solubility of Phosphorus in Feces from Lactating Dairy Cows." J. Dairy Science 328:2598–611.
Nair, V. D., D. A. Graetz, and K. M. Portier. 1995. "Forms of Phosphorus in Soil Profiles from Dairies of South Florida." Soil Sci. Soc. Am. J. 59:1244–49.
Nair, V. D., D. A. Graetz, and D. O. Dooley. 2003. "Phosphorus Release Characteristics of Manure and Manure-impacted Soils." J. Food Agric. Environ. 1:217–23.
Nair, V. D., W. G. Harris, D. Chakraborty, and M. Chrysostome. 2010. Understanding Soil Phosphorus Storage Capacity. SL336. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/ss541.
Nair, V. D., M. Chrysostome, and W.G. Harris. 2011. The Long-term Contribution of Phosphorus from Agricultural Lands to Lake Okeechobee. SL357. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/ss558.
Acknowledgments
This study was supported in part by a grant from the Florida Department of Agriculture and Consumer Services. The authors thank Tom Obreza and Ed Hanlon for their helpful comments and suggestions on the draft version of this document.