This publication is part of a series titled Contaminants in the Urban Environment. This series is intended to give state and local government officials, soil scientists, consulting engineers, Extension agents, and citizens (1) a basic understanding of the occurrence, toxic effects, and source of various contaminants in the environment and (2) guidance on ways to protect human and environmental health.
Introduction and Purpose
Perfluoroalkyl substances (PFASs) are also known as perfluorochemicals (PFCs). Understanding the existence of PFASs in the environment is important because they are the most widespread and persistent manmade chemicals on earth. Some of the most common products that contain PFASs are Teflon pans, non-stick cookware, rain/waterproof jackets (like Gore-Tex), fire-fighting foams, food packaging, carpets, and furniture fabrics. PFASs stay in the environment for a long period of time. This long environmental residence time means that, like other persistent chemicals (such as DDT), PFASs can accumulate in organisms to levels that cause harmful effects.
PFASs are present in many environmental matrices, such as water, sediments, and wildlife (Houde et al. 2011). They are harmful (and potentially toxic) to vertebrates such as fish, birds, and mammals at certain concentrations. Two PFASs—perfluorooctanoic acid (PFOA) and perfluorooctanoic sulfonate (PFOS)—are so widespread that they are believed to be present in the blood, breast milk, kidneys, and liver of every person (Kannan et al. 2004).
The many existing types of PFASs are grouped according to the number of carbon atoms present in the structure. Here we focus on the most commonly found PFASs in the environment, such as PFOA and PFOS (which are linear perfluoroalkyls that have 5 to 14 carbon atoms).
Some researchers state that adverse effects of PFASs on the general human population are very unlikely due to the low levels of exposure (Stahl et al. 2011). For example, general population exposure can be 100 times lower than the occupational exposures at the workplaces where PFASs are manufactured and added to products (Olsen et al. 1999). However, toxicological work on laboratory animals indicates that PFASs can cause potential effects on development (growth) (Du et al. 2009), reproduction (Oakes et al. 2004), and suppression of the normal immune response (Yang et al. 2002). Moreover, PFASs are potential carcinogenic (cancer-causing) compounds (Jacquet et al. 2012). Therefore, we need to know the sources and toxicity potential of PFASs in order to protect ourselves and the environment from PFASs.
The purpose of this document is to discuss the occurrence, use, exposure, and potential harmful effects of PFASs to humans and the environment, and to suggest potential solutions for reducing PFAS exposure.
What are perfluoroalkyl substances?
Perfluoroalkyl substances are manmade organic chemicals formed by a carbon backbone where all the hydrogen atoms are replaced with fluorine atoms (Figure 1). Hundreds of types of PFASs exist. Some have short backbones (fewer than five carbon atoms) that tend to volatilize (evaporate), others are more stable (they do not evaporate) and have a long chain (5 to 14 carbons) like PFOS and PFOA. More stable PFASs are widespread in the environment because they do not degrade.
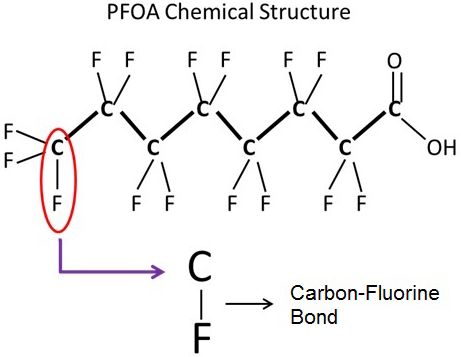
Credit: Ignacio A. Rodriguez-Jorquera, UF/IFAS
The presence of the carbon–fluorine bond gives PFASs extraordinary stability over a wide heat range and chemical stability, as this bond is considered the strongest single bond in organic chemistry. The strength of this bond is a large reason why these compounds are so widely used, but it is also why PFASs do not break down and are extremely persistent in the environment.
What are the sources of PFASs in the environment?
A few of the most well-known sources of PFASs are Teflon pans and non-stick cookware (Figure 2). PFASs have both hydrophobic (water-hating) and hydrophilic (water-loving) ends and are used to resist stains in products such as Scotchgard. Many products—clothing, rain/waterproof jackets (like Gore-Tex), fire-fighting foams, food packaging, carpets and furniture fabrics, and pesticides—also contain PFASs. Automobile, aerospace, and electronics industries use PFASs because of their excellent surfactant properties such as reducing friction and improving the efficiency of engines.

Credit: iStock/Thinkstock.com (non-stick pan, waterproof textile, and fire fighting foam)/Digital Vision/Thinkstock.com (fast food)
Production of the main PFASs in the United States has been declining since 2002 because 3M Company, the main manufacturer of PFOS, completed a voluntary phase-out. Another PFAS called PFOA is now produced in reduced amounts and eight major producers of PFOA voluntarily agreed to phase out production by 2015 (US EPA 2017). However, other PFASs are imported and have specific limited uses. PFOS and PFOA can be produced as byproducts of their parent compounds through degradation, and these byproducts are present in many products. So although phasing out is good to eliminate future sources, it does not mean that these compounds are completely gone from the environment. Like other persistent contaminants such as DDT and PCBs, the legacy of PFASs may be with us for some time to come.
Why are PFASs widespread in the environment?
PFASs are widespread because they are used in many daily life applications. They are released to the environment (1) indirectly as a result of environmental degradation of parent compounds that are now widespread in human usage and/or (2) directly through manufacturing and consumer products (Prevedouros et al. 2005).
The two main PFASs, which are PFOS and PFOA, are found in the environment because they do not degrade or convert to other compounds (Ahrens 2011; Gladysz and Jurisch 2012). Thus, like many other human-made contaminants, PFASs end up in soil and water bodies. The occurrence and behavior of PFASs in lakes, streams, and rivers are recognized as an emerging issue of concern.
The impact of PFASs on wildlife has not yet been established. However, PFASs have been detected in many types of wildlife living in remote areas, such as polar bears in the Artic and marine birds in the Antarctic (Houde et al. 2011). Since PFASs mostly originate in urban locations, it is not surprising that water bodies in urban areas tend to have higher occurrence of PFASs. Thus, aquatic organisms in urban water bodies may be more prone to accumulate PFASs and to exhibit any potential deleterious effects.
Because vertebrates (including humans) cannot metabolize PFOS and PFOA (Stahl et al. 2011), it is very difficult and slow for the human body to get rid of these compounds. It is estimated that humans need about three years to eliminate half of the PFASs concentrations present in the body (Olsen et al. 2007).
How can you be exposed to PFASs?
You can be exposed to PFASs via oral, dermal (skin), or inhalation routes. Oral uptake results in rapid and almost total (95%) assimilation in the body (Kudo and Kawashima 2003). Considering the popularity of the products that contain PFASs, the most common exposure routes for humans are food, cookware, water, and household dust, as shown in Figure 3 (Stahl et al. 2011).
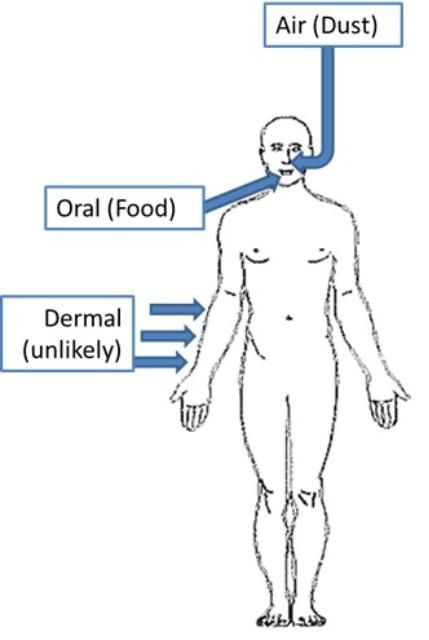
Credit: Ignacio Rodriguez-Jorquera, UF/IFAS
Oral Intake: In adults, the exposure to PFOA and PFOA can occur from food grown in PFAS-contaminated soil (e.g., potatoes, corn, and wheat) and from consumption of fish, meat, or milk from animals previously exposed to PFASs (Stahl et al. 2011). Transfer of PFASs from food that has been in contact with paper and cardboard treated with PFASs (for impermeability) has been also reported, as well as transfer from non-stick cooking utensils (Stahl et al. 2011). Exposure from drinking water and other aquatic sources are also important sources of exposure. For instance, fish and water account for 90% of total dietary PFOS exposure in humans (Stahl et al. 2011).
PFASs may also enter the body due to the ingestion of dust from household products that contain PFASs, such as carpets, shoes, furniture, or textiles (Stahl et al. 2011). Estimation of this exposure route is difficult because there are a large number of products that contain PFASs. Some authors consider this route negligible as compared to food intake (Stahl et al. 2011).
Presence of PFOS and PFOA in breast milk has been demonstrated from studies in several countries. Postnatal exposure for infants can be especially significant from breast milk in Western and highly industrialized countries (Fromme et al. 2009).
Dermal Intake: Studies performed in rodents suggested that dermal intake of PFASs is possible but less relevant than oral exposure.
Inhalation Intake: Insufficient data are currently available with regard to PFASs intake via inhalation, so the amount of PFASs that enters the body through inhalation cannot be stated. It is clear that household dust can contain fairly high amounts of PFASs (Kato et al. 2009). Nevertheless, studies that used models to estimate this exposure determined that inhalation intake is the least probable route of exposure, being 100 times less than oral exposure.
What are the effects of PFASs on animal and human health?
Negative effects of PFASs on laboratory animals—from both short-term (acute) exposures of high concentrations and long-term (chronic) low-concentration exposures—have been reported. Studies in humans are limited to relationships between diseases/behavior problems and relatively high concentrations of PFASs in blood, but the available studies remain insufficient to declare PFASs as the cause of diseases in humans. Nevertheless, some evidence suggest that PFASs could alter thyroid hormones levels (key hormone for metabolism regulation) in pregnant woman and infants (Wang et al. 2014).
In general, various effects—including the alteration of lipid (fats) metabolism, changes in the thyroid and immune system, developmental effects, and hormonal effects—have been demonstrated in different classes of laboratory and wildlife animals (Liu et al. 2007). In laboratory animals, PFASs can perturb lipid metabolism and transport, with contrasting findings related to the ability of PFASs to increase or reduce cholesterol levels in plasma.
Interestingly, researchers have associated high levels of cholesterol in human blood with high level of PFASs (Eriksen et al. 2013), but the mechanism by which these two variables are connected is unclear. With simple associations being the only evidence, it is not possible to assume that PFASs are causing the harmful effect.
Cancer has been observed in the liver, reproductive organs, and pancreas in laboratory animals chronically exposed to PFOS (Stahl et al. 2011). In rats exposed to PFASs during pregnancy, developmental effects such as reduced weight at birth and reduced number of live births have been reported. Recently, the effects on nervous system (neurotoxicity) and behavioral changes (hyperactivity) have been added to the list of deleterious effects caused by PFASs in lab animals (Mariussen 2012).
What are the impacts of PFASs on the environment?
The potential deleterious effects caused by PFASs on wildlife animals and aquatic biota include estrogenicity (feminization), alteration of sex hormone production, reduced egg production in fish, alteration of testicular architecture, depression of the immune system, and toxic effects on liver (Houde et al. 2011).
Aquatic ecosystems (such as lakes, streams, rivers, and estuaries) are particularly vulnerable to PFAS contamination. For example, wastewater treatment plants (WWTPs) are not able to reduce or remove PFASs in water or activated sludge (biosolids). In fact, due to the transformations of PFAS precursor compounds by bacteria in the WWTPs, the concentrations of PFASs in outgoing wastewater are actually elevated compared with incoming sources. Deleterious effects of PFASs have been observed in several aquatic organisms from invertebrates to fish.
Researchers have shown that one type of volatile PFASs, perfluorotributylamine (PFTBA), can contribute to global warming because in the atmosphere this compound can hold more heat than carbon dioxide (CO2). Further, the inherent persistence of PFASs makes their contribution to the greenhouse effect greater than CO2 because PFASs stay in the atmosphere for a longer period of time (Hong et al. 2013).
Best Practices to Protect Yourself and the Environment from PFAS Exposure
- The best way to protect yourself is to reduce the potential exposure to PFASs as much as possible. First, minimize the purchase and use of products that contain PFASs. For example, cooking implements and pans that do not contain PFOA are available on the market. Second, reducing the consumption of food or the use of products containing PFASs might also lower the risk of exposure.
- Reduce the consumption of prepared food. For example, microwave popcorn or pizza has been listed as a significant source of PFASs because the interior coating of the packages contains PFASs.
- Avoid buying clothing and furniture products labeled as stain resistant, since most of these contain PFASs.
- In 2010, the EPA issued drinking water provisional health advisories of 0.4 μg/L PFOA and 0.2 μg/L PFOS, as these have been found in some drinking water samples in the country. Thus, consumers may want to use carbon-filtered water to avoid potential exposure to PFASs from drinking water.
By reducing the use of products containing PFASs, you will also be helping to reduce these chemicals in the environment, as domestic use of products is the main source of PFASs in the environment. The breakdown of products containing PFASs is also a source of these contaminants in the environment, such as in dust, water (from your home to rivers and streams, since WWTPs cannot remove them), and soils.
Summary
PFASs comprise a group of highly resistant manmade chemicals that can have deleterious effects on the environment and possibly on humans. The production of PFASs in the United States has been reduced, but they are still present in many domestic and industrial applications. For humans, main routes of PFAS exposure are diets containing fish and prepared food (packaging material). Water can also be an important source in areas with high concentration of PFASs in drinking water. Among all PFASs, PFOS is the most common and represents the greatest concern to human health and environment. Toxicity to liver and lipid metabolism alteration are the most frequently reported toxic outcomes from experimental tests. Cancer (tumor growth) has been observed in experimental animals chronically exposed to PFASs. Recent immunotoxic and neurotoxic findings in laboratory animals call for further epidemiological research in humans. Most of the current research is from laboratory animals exposed to single PFASs (mainly PFOS). Thus, a complete assessment of current risk considering mixtures of PFASs is not yet possible. In the meantime, to reduce self-exposure and reduce your PFASs contribution to the environment, avoid use of products containing PFASs in households and avoid the consumption of food that has been in contact with PFASs via their packaging (e.g., microwave popcorn and pizza).
Further Reading
For more detailed information on PFASs, the following articles are recommended:
Ahrens, L. 2011. "Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate." Journal of Environmental Monitoring 13: 20–31.
Houde, M., A. O. De Silva, D. C. G. Muir, and R. J. Letcher. 2011. "Monitoring of Perfluorinated Compounds in Aquatic Biota: An Updated Review." Environmental Science & Technology 45: 7962–7973.
Rodriguez-Jorquera, I., K. J. Kroll, G. S. Toor, and N. D. Denslow. 2015. "Transcriptional and Physiological Response of Fathead Minnows (Pimephales promelas) Exposed to Urban Waters Entering into Wildlife Protected Areas." Environmental Pollution 199: 155–165. DOI: 10.1016/j.envpol.2015.01.021.
Stahl, T., Mattern, D., Brunn, H. 2011. "Toxicology of perfluorinated compounds." Environmental Sciences Europe 23:38, 1–52.
References
Ahrens, L. 2011. "Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate." Journal of Environmental Monitoring 13: 20–31.
Du, Y., X. Shi, C. Liu, K. Yu, and B. Zhou. 2009. "Chronic effects of water-borne PFOS exposure on growth, survival and hepatotoxicity in zebrafish: A partial life-cycle test." Chemosphere 74: 723–729.
Eriksen, K. T., O. Raaschou-Nielsen, J. K. McLaughlin, L. Lipworth, A. Tjønneland, K. Overvad, and M. Sørensen. 2013. "Association between Plasma PFOA and PFOS Levels and Total Cholesterol in a Middle-Aged Danish Population." PloS ONE 8, e56969.
Fromme, H., S. A. Tittlemier, W. Völkel, M. Wilhelm, and D. Twardella. 2009. "Perfluorinated compounds—Exposure assessment for the general population in western countries." International Journal of Hygiene and Environmental Health 212: 239–270.
Gladysz, J., and M. Jurisch. 2012. "Structural, Physical, and Chemical Properties of Fluorous Compounds," in Fluorous Chemistry, edited by I. T. Horváth. Springer Berlin Heidelberg, pp. 1–23.
Hong, A. C., C. J. Young, M. D. Hurley, T. J. Wallington, and S. A. Mabury. 2013. "Perfluorotributylamine: A novel long‐lived greenhouse gas." Geophysical Research Letters 40(22): 6010–6015.
Houde, M., A. O. De Silva, D. C. G. Muir, and R. J. Letcher. 2011. "Monitoring of Perfluorinated Compounds in Aquatic Biota: An Updated Review." Environmental Science & Technology 45: 7962–7973.
Jacquet, N., M. A. Maire, Y. Landkocz, and P. Vasseur. 2012. "Carcinogenic potency of perfluorooctane sulfonate (PFOS) on Syrian hamster embryo (SHE) cells." Archives of Toxicology 86: 305–314.
Kannan, K., S. Corsolini, J. Falandysz, G. Fillmann, K. S. Kumar, B. G. Loganathan, M. A. Mohd, J. Olivero, N. V. Wouwe, J. H. Yang,, and K. M. Aldous. 2004. "Perfluorooctanesulfonate and Related Fluorochemicals in Human Blood from Several Countries." Environmental Science & Technology 38: 4489–4495.
Kato, K., A. M. Calafat, and L. L. Needham. 2009. "Polyfluoroalkyl chemicals in house dust." Environ Res 109: 518–523.
Kudo, N., and Y. Kawashima. 2003. "Toxicity and Toxicokinetics of Perfluorooctanoic Acid in Humans and Animals." The Journal of Toxicological Sciences 28: 49–57.
Liu, C., Y. Du, and B. Zhou. 2007. "Evaluation of estrogenic activities and mechanism of action of perfluorinated chemicals determined by vitellogenin induction in primary cultured tilapia hepatocytes." Aquatic Toxicology 85: 267–277.
Mariussen, E. 2012. "Neurotoxic effects of perfluoroalkylated compounds: mechanisms of action and environmental relevance." Archives of Toxicology 86: 1349–1367.
Oakes, K. D., P. K. Sibley, K. R. Solomon, S. A. Mabury, and G. J. Van Der Kraak. 2004. "Impact of perfluorooctanoic acid on fathead minnow (Pimephales promelas) fatty acyl-coa oxidase activity, circulating steroids, and reproduction in outdoor microcosms." Environmental Toxicology and Chemistry 23: 1912–1919.
Olsen, G. W., J. M. Burris, D. J. Ehresman, J. W. Froehlich, A. M. Seacat, J. L. Butenhoff, and L. R. Zobel. 2007. "Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers." Environmental Health Perspectives 115: 1298–1305.
Olsen, G. W., J. M. Burris, J. H. Mandel, and L. R. Zobel. 1999. "Serum Perfluorooctane Sulfonate and Hepatic and Lipid Clinical Chemistry Tests in Fluorochemical Production Employees." Journal of Occupational and Environmental Medicine 41: 799–806.
Stahl, T., Mattern, D., Brunn, H. 2011. "Toxicology of perfluorinated compounds." Environmental Sciences Europe 23:38, 1–52.
US EPA. 2017. Technical Fact Sheet–Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA). Available at: https://19january2021snapshot.epa.gov/sites/static/files/2017-12/documents/ffrrofactsheet_contaminants_pfos_pfoa_11-20-17_508_0.pdf. Accessed on 24 March 2022.
Wang, Y., W. J. Rogan, P. C. Chen, G.-W. Lien, H. Y. Chen, Y. C. Tseng, M. P. Longnecker, and S. L. Wang. 2014. "Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study." Environmental Health Perspectives 122: 529–534.
Yang, Q., M. Abedi-Valugerdi, Y. Xie, X.-Y. Zhao, G. Möller, B. Dean Nelson, and J. W. DePierre. 2002. "Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid." International Immunopharmacology 2: 389–397.