Most people agree that healthy, well-maintained turfgrass is a thing of beauty. However, many of these same people think beautiful turfgrass requires a lot of trouble, hard work, and possible expertise that they do not possess. This is not necessarily true, but a few basic facts concerning the nutritional requirements of turfgrasses and the properties of fertilizer and liming materials are essential for growing healthy turfgrass. Under normal conditions, water, light, and temperature have greater influences on turfgrass growth and quality than nearly any other variable. However, when water, light, and temperature are at optimal levels and the turfgrass remains unacceptable, nutrient deficiencies may be the cause. Therefore, it is essential to understand how soil testing may be used to more efficiently manage nutrient applications.
Soil Analysis and Interpretation
Soil testing involves sample collection, analysis, interpretation, and recommendations. Sample collection is the first step of soil analysis. The soil test and the resulting recommendations represent the soil nutrient status only as well as the sample does. Therefore, it is imperative that the soil sample be taken and handled properly. Soil samples should be taken from the soil depth in which the majority of turfgrass roots exist, which is normally the top four inches of the soil. The soil samples can be taken using a traditional soil probe, a garden spade, or shovel. Ten to fifteen samples should be randomly taken from the area of concern. Do not mix soil from healthy turf areas with soil from unhealthy turf areas because this will reduce the ability to diagnose the problem. The 10–15 samples should be mixed, and a one-pint portion placed in a soil sample bag and shipped to any soil testing laboratory. Soil sample bags can be acquired from the UF/IFAS extension soil testing laboratory (ESTL) at https://soilslab.ifas.ufl.edu/. The UF/IFAS ESTL also performs soil testing for a fee.
The second step in soil analysis is extraction. The purpose of the extractant is to determine the quantity of an element that would be representative of what will be available for plant uptake during that growing season. Following extraction, the extracted solution from the soil is introduced into an instrument that detects the element concentrations.
The third step of soil testing is data interpretation. The nutrient concentrations are interpreted by comparing the nutrient concentration to known nutrient response curves for the given soil and the turfgrass of interest. Unfortunately for Florida turfgrasses, nutrient response calibration curves do not exist for phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), and all micronutrients. Until calibration curves are determined, the use of soil testing to manage the application of nutrients to turfgrasses (other than for pH or salinity) is questionable (Kreuser, 2015). However, critical soil test values have been determined for P and Mg and are 10 and 20 ppm, respectively (Liu et al. 2008; Sartain 1993). Critical soil test values only provide a single value above which no plant response would be expected, but do not provide any response probability when soil test results are below the critical value.
The final step is to provide a recommendation based upon the interpretation. As previously mentioned, because calibration curves for each Florida turfgrass and each soil type do not exist, the use of soil testing to manage nutrients applied to Florida turfgrasses are of little value. However, the critical soil test values for P and Mg can be used to recommend that no P or Mg be applied when soil test values exceed 10 and 20 ppm, respectively.
Soil Acidity
So, if soil testing has limited value for managing turfgrass nutrient applications, why should you test the soil? Simply, because pH and salinity are of great value and are evidence-based components of most soil tests. Soil reaction, or pH, is important because it influences several soil factors that affect plant growth. Soil bacteria that transform and release N from organic matter function best in the pH range of 5.5 to 7.0; certain fertilizer materials also supply nutrients more efficiently in this range.
Plant nutrients, particularly P, K, Ca, Mg, boron, copper, iron, manganese, and zinc, are generally more available to plants in the pH range of 5.5 to 6.5 (McBride 1994). Some of these plant nutrients are more available to plants at pH values below 5.0 than in soils with pH between 5.0 and 7.5. In certain soils, when the soil pH drops below 5.0, aluminum may become toxic to plant growth.
Turfgrasses differ in their adaptability to soil acidity. For example, centipedegrass and bahiagrass grow better in an acid environment (pH 5.0 to 6.0) than St. Augustinegrass or zoysiagrass, which grow best in near neutral or alkaline soils (pH 6.5 to 7.5) (Figure 1).
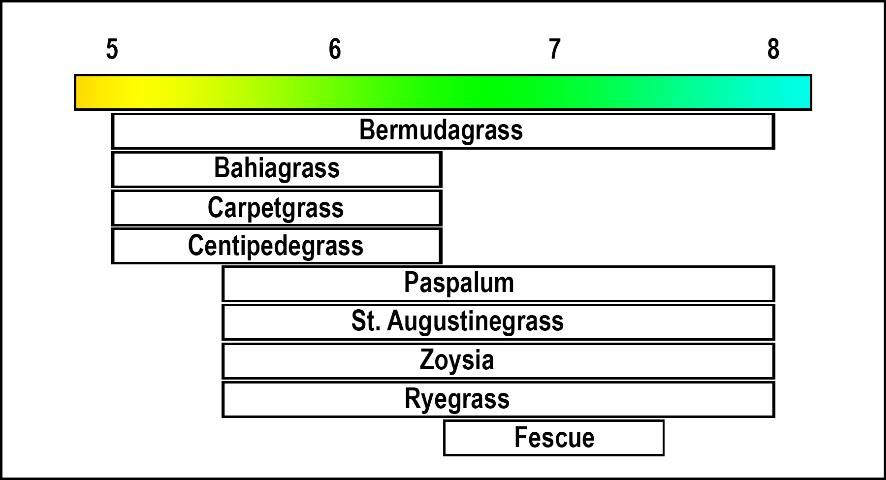
Adjusting the Soil Reaction (pH)
Normally, liming materials are used to increase soil pH and supply the essential nutrients Ca and Mg. The two most commonly available liming materials are calcic and dolomitic limes (Table 1). Generally, about 6 months' reaction time is required for calcic and dolomitic lime to have their maximum effect on soil acidity. If more immediate results are desired, hydrated lime can be used; however, hydrated lime is not recommended for use by the non-professional because this material can severely damage the turfgrass if improperly used. Lime recommendations are typically made on a calcic limestone basis. If another liming material is used, adjust the application rate according to the calcium carbonate equivalents (Table 1).
Soil lime requirement cannot be determined by soil pH alone. Liming recommendations are based on the Adam-Evans lime requirement test, which is included in routine soil analysis, and is reported if the soil pH is 6.0 or less. The quantity of lime recommended is based on the type of turfgrass being grown, the target pH desired, and is highly dependent upon soil type. The greater the amount of organic matter or clay content of the soil and the lower the pH, the more lime is required to increase the soil pH to a desired level.
Soil Alkalinity
If a soil is too alkaline (has a pH greater than 7.5), it must be determined whether the excess alkalinity is due to an inherent soil characteristic or previous excessive application of liming materials. Soils having a pH greater than 8.3 are not alkaline due to the presence of calcium carbonate materials because calcium carbonate has an equilibrium pH of 8.3 in water. Thus, excessively high soil pH is most likely due to the presence of elevated levels of sodium (Na). It is difficult and uneconomical to change the pH of naturally occurring alkaline soils, such as those found in coastal areas or fill soil containing marl, shell, or limestone. On the other hand, if a high pH is due to applied lime or other alkaline additives, then acid-forming materials such as sulfur and ammonium sulfate (Table 2) can effectively reduce soil pH when applied at the proper rate and frequency.
Granular, super-fine dust, or wettable sulfur can be used to decrease soil pH. Granular sulfur is preferred on turfgrass production systems due to the ease of application (with cyclone fertilizer spreaders) and the reduced possibility of foliar burn from the granules. Thoroughly water-in sulfur after application, taking care to wash off all above ground turf parts. It takes approximately 1/3 the amount of sulfur to decrease the soil pH 1 unit as it does calcic lime to increase the soil pH 1 unit. Do not apply more than 10 pounds of sulfur per 1000 square feet per application. Additional applications of sulfur should not be made more often than once every 30 days. Depending on the quantity of excess lime in the soil, several applications of sulfur may be necessary to decrease the soil pH to the desired level. However, as stated above, if the soil is inherently high in pH due to the natural presence of lime in the soil, pH cannot be reduced over a long period of time and will gradually rebound. If the soil has a naturally high calcium carbonate content, it would be more practical and much easier to change to a type of turfgrass that will tolerate high soil pH and not attempt to reduce the soil pH using a sulfur-containing material. Sulfur oxidizes in the soil and reacts with water to form a strong acid (sulfuric acid) that can severely damage plant roots, so it must be used cautiously.
Soil Salinity
At high enough levels, soil salts deplete the turfgrass's moisture and can result in stressed and unacceptable turfgrass. Symptoms of salinity stress in turfgrass looks extremely like those of drought. Salts are introduced to turfgrass soils via many channels, including but not limited to irrigation water, effluent water, ocean spray, saltwater intrusion, and fertilizer (Carrow et al., 1999). Soils may require remediation if the soil salt level exceeds the tolerance of the turfgrass being grown (Table 3).
The most effective method of reducing soil salts is to rely upon natural rainfall and/or low-saline irrigation. If clean water is not available, irrigate deeply with existing water to leach the salts remaining in the rootzone from prior irrigation cycles. Despite claims, gypsum (CaSO4) will not remove salts and does little to increase percolation in Florida's sandy soils. Gypsum is a salt and, therefore, the application of gypsum increases salinity. However, gypsum can remediate Na-related issues such as poor percolation resulting from deflocculated soils. Florida's soils are predominately sands, which have a very poor capacity to retain Na and, therefore, Na-related soil problems are rare. However, gypsum has the additional benefit of reducing bicarbonates and carbonates (Figure 2), which can be toxic to many turfgrasses (Carrow et al. 2001).
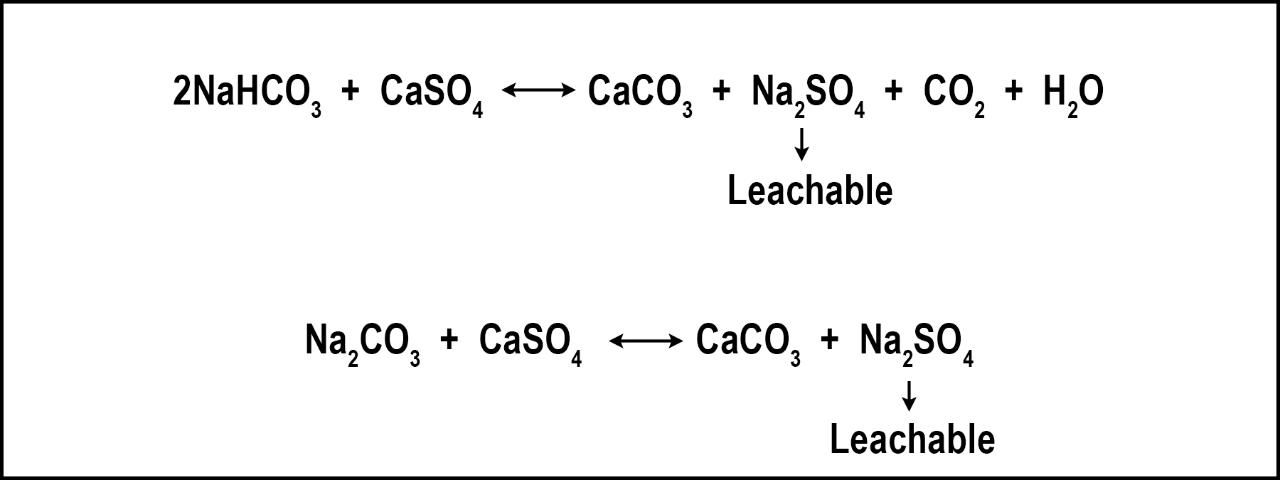
In locations where Na and/or bicarbonates are continually added to the turf/soil system, remediation may require regular gypsum applications over several years, in some cases. On established turf, gypsum application rates range from 200-500 lbs. per acre. If your normal fertilizer contains filler, your fertilizer distributor should be able to replace the filler with gypsum. In this manner, gypsum applications would then be a regular part of your nutrient applications with very little appreciable increase in cost. This method may be less expensive than sole gypsum applications, but it will require more time to achieve the same remediation effect.
Summary
Soils should be regularly tested for pH and salinity levels. In many cases, turfgrass issues can be resolved by adjusting the pH into the range of 5.0-6.5. Salinity levels should be compared to the tolerance level of the turfgrass being grown. If diagnosed as an issue, bicarbonates and carbonates can be reduced by applying gypsum. Turfgrass response to P and Mg fertilizers is unlikely when Mehlich III soil test values exceed 10 and 20 ppm, respectively.
References
Carrow, R.N. and R.R. Duncan. 1998. Salt-affected turfgrass sites: assessment and management. Chelsea, Mich: Ann Arbor Press.
Carrow, R.N., R.R. Duncan, and M. Huck. 1999. "Treating the cause, not the symptoms: Irrigation water treatment for better infiltration". USGA Green Section Record. 37: 11–15.
Carrow, R.N., D.V. Waddington and P.E. Rieke. 2001. Turfgrass soil fertility and chemical problems: assessment and management. Ann Arbor Press, Chelsea, Mich.
Kreuser, W.C. 2015. "Simplifying soil test interpretations for turf professionals". G2265: https://turf.unl.edu/sites/unl.edu.ianr.agronomy-horticulture.turf/files/media/file/Simplifying-Soil-Test-Interpretations-g2265.pdf
Liu, M., J.B. Sartain, L.E. Trenholm, and G.L. Miller. 2008. "Phosphorus requirements of St. Augustinegrass grown in sandy soils". Crop Sci. 48: 1178-1186. doi:10.2135/cropsci2007.09.0506.
McBride, M.B. 1994. Environmental chemistry of soils. New York: Oxford University Press.
Sartain, J.B. 1993. "Interrelationships among turfgrasses, clipping recycling, thatch, and applied calcium, magnesium, and potassium". Agron. J. 85: 40–43.