Introduction
Strawberries grown in Florida are attacked by several arthropod pests, including flower thrips. Western flower thrips (Frankliniella occidentalis) (Fig. 1) and common blossom thrips (F. schultzei) are invasive species of thrips that have the potential to cause damage to strawberries in Florida (Pinent et al. 2011; Mossler 2013). Florida flower thrips (F. bispinosa) is a species of thrips native to Florida that is commonly found in strawberry blossoms. This species has the ability to cause economic damage to blueberries; however their ability to cause economic damage to strawberry has not been established (Rhodes et al. 2012; Frantz and Fasulo 2013).

Credit: JD Cluever
Thrips are small insects ranging from 1 to 4 millimeters in length. They have piercing-sucking mouthparts which are used to ingest the cellular liquids of plants (Hunter and Ullman 1989). The life stages of thrips in the suborder Terebrantia include the egg, larva I, larva II, prepupa, pupa, and adult. The female terebrantian thrips possess a saw-like ovipositor which is used to insert eggs into the foliage of a host plant. Larval and adult thrips are typically found feeding in concealed spaces of the plant and the prepupal and pupal stages are found in the soil (Morse and Hoddle 2006).
Thrips have a short generation time. At 77°F it takes 10 to 16 days for these flower thrips to develop from an egg to adult. As the temperature gets cooler the amount of time it takes to reach adulthood increases (Milne and Walter 1997; MacDonald et al. 1998; Bi-Song 2001). Thrips populations have the potential to increase rapidly. For example, a Florida flower thrips female can produce over 120 eggs in her life time at 77°F when developing on Alocasia cucullata leaves and cattail pollen (Bi-Song 2001). A western flower thrips female can lay over 80 eggs in her lifetime at 77°F when feeding on pepper pollen and sucrose (Nielson et al. 2010). A common blossom thrips female is able to produce about 60 eggs at 77°F when fed a larval diet of Malvaviscus arboreus and sucrose (Milne et al. 1996). Information about thrips reproduction on strawberry is lacking. Thrips are able to reproduce without mating. Most species are arrhenotokous, which means males are produced from unfertilized eggs and females from fertilized eggs. Thrips can disperse rapidly by taking advantage of air currents to colonize new areas (Lewis 1991; Kumm and Moritz 2009).
Many thrips have a wide host range. However, not all plants are suitable for reproduction of thrips. Pepper is an example of a good reproductive host for both western flower thrips and Florida flower thrips, and tomato is an example of a poor reproductive host (Brodbeck et al., 2001; Avila et al., 2006). High numbers of thrips adults may still be found feeding on poor reproductive hosts, for example when migration into the field is high (Funderburk et al. 2013). Some examples of weeds that may be found around Florida strawberry fields that are hosts to thrips are Bidens spp. (Spanish needle) and Raphanus raphanistrum (wild radish). Note that the majority of the thrips in these weeds in Florida are Frankliniella bispinosa. The beneficial predator Orius spp. (minute pirate bug) is also commonly found in Bidens spp. (Frantz and Mellinger, 1990; Eger et al. 1998; Bottenberg at al. 1999; Rhodes and Liburd 2011).
Damage
Less than 2% of known thrips species are considered pests. Most of these pest thrips are in the family Thripidae. In strawberries, thrips are implicated in causing flower and fruitlet abortion, petal browning, necrotic flecking, and distorted, bronzed fruit (Fig. 2). Feeding on the flowers can cause withering of anthers and stigmas, resulting in non-uniform fertilization which produces malformed fruit. Feeding on the fruit may result in cracking, bronzing, and a reduction in fresh weight (Coll et al. 2005; New Brunswick 2014; Zalom et al. 2014). Bronzing can also be caused by factors other than thrips including cyclamen mites and spray applications (Polito et al. 2002).

Credit: H. A. Smith
Thrips numbers can be quantified easily by examining flowers from the field. Scouting can be accomplished in different ways. One method is to use a 10X hand lens to examine the blooms for the presence of thrips. Another method is to place a sample of blooms in alcohol and then count the numbers with the use of a microscope (Frantz and Fasulo, 2013). However, recommended action thresholds for thrips vary widely from as low as 3-11 per flower to as high as 50 per flower for various species (Steiner and Goodwin 2005). It is not easy to determine which species of thrips are present in a field. However, different species may differ in their ability to inflict damage.
Frankliniella bispinosa (Florida Flower Thrips)
This species is believed to be native to Florida and it is found in Florida, Georgia, Alabama, the Bahamas, Bermuda, Colombia, Mexico, Japan, Taiwan, and Trinidad (Hoddle et al. 2012; Martin et al. 2012) . It is the most common species in many crops (including strawberry) and weeds in central and south Florida. This species is also found in abundance in north Florida but is less dominant than the eastern flower thrips (F. tritici). Problems may occur when high numbers of these thrips migrate from citrus groves following bloom or from oak trees. When treatment is necessary, insecticides that have short residual activity are recommended (Frantz and Fasulo 2013).
Frankliniella occidentalis (Western Flower Thrips)
This species is a native of western North America, but has spread throughout the world. It was first discovered in Florida in the early 1980's (Kirk and Terry, 2003). This species is known to be a pest of many crops worldwide. It is most common in north Florida, but is present throughout the state. This species can be difficult to control due to its tendency to develop resistance to insecticides. Its prevalence may increase when broad-spectrum insecticides such as pyrethroids are used. Difficulties managing western flower thrips may be due to resistance to insecticides, reduction in numbers of predators like Orius sp., or the removal of F. bispinosa as a competitor. (Hansen et al., 2003; Frantz and Fasulo 2013).
Frankliniella schultzei (Common Blossom Thrips)
This species is native to Africa but is now found throughout the world in the tropics and subtropics. In Florida this species is most prevalent in the southern portion of the state (Galen Frantz, unpublished data). Incidence is more frequent when broad-spectrum insecticides such as pyrethroids are used. (Frantz and Fasulo 2013). Both the brown and yellow morphs exist in Florida, although the dark morph is more common.
Minor Thrips Species
Frankliniella fusca (tobacco thrips) is found occasionally in central Florida strawberries; however, it is more prevalent in the northern portion of the state. Scirtothrips dorsalis (chilli thrips) may damage strawberry in some years (Galen Frantz, unpublished data). Thrips tabaci (onion thrips) is also found occasionally in strawberries. Another species encountered on occasion is the spider mite predator Scolothrips pallidus (Gilstrap and Oatman 1976).
Characteristics to Distinguish among F. bispinosa, F. occidentalis and F. schultzei
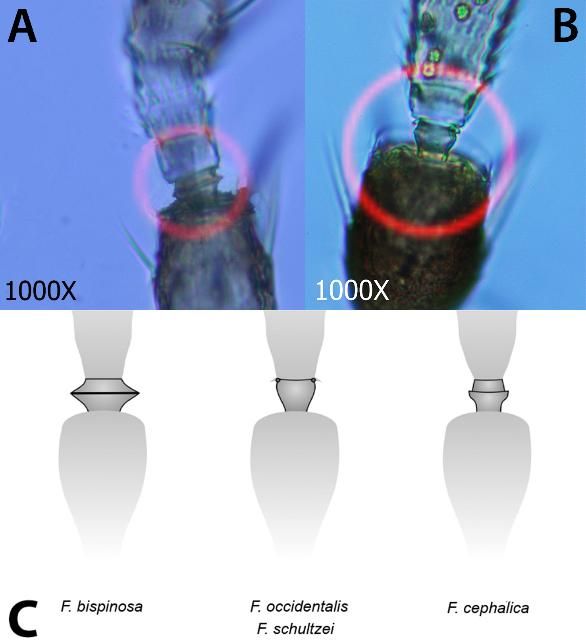
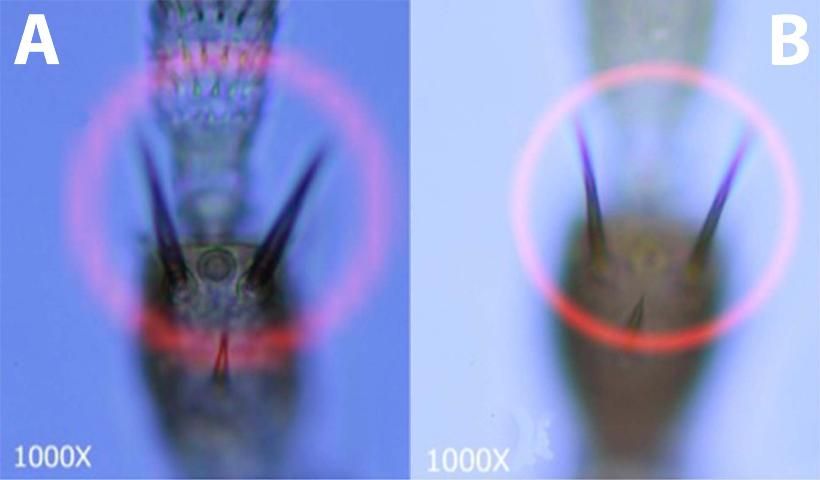
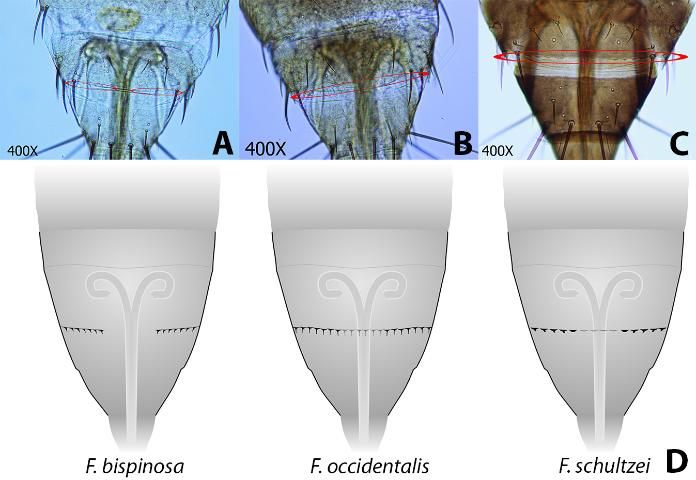
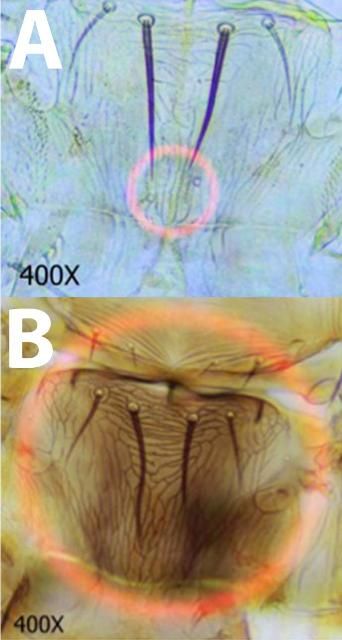
- 1. Pedicel of third antennal segment flange-shaped (fig. 3a., Fig. 3c.); Setae arising from second antennal segment forming thick, heavy spines (Fig 4a.); Microtrichial comb on tergite VIII well developed and interrupted in center (Fig. 5a., Fig. 5d.).................................................Frankliniella bispinosa
- 1'. Pedicel of third antennal segment smooth (Fig 3b., Fig. 3c.); Setae arising from second antennal segment not forming thick, heavy spines (Fig 4b.); Microtrichial comb on tergite VIII well developed and not interrupted or not well developed (Fig 5b., 5d.)..................................... Go to 2
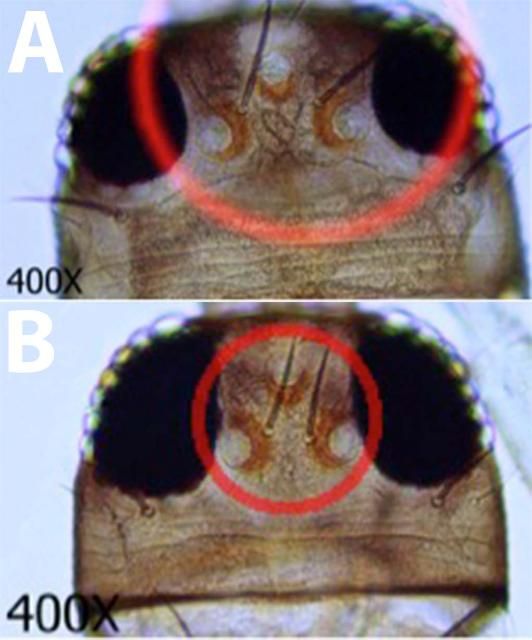
- 2. Interocular setae (commonly referred to as Ocular III setae) arising far apart (Fig. 6a.); metanotal campaniform sensillae usually present (Fig. 7a.); Microtrichial comb on tergite VIII well developed (Fig. 5b., 5d.) ...... Frankliniella occidentalis
- 2'. Interocular setae arising close together (Fig 6b.); metanotal campaniform sensillae absent (Fig. 7b.); Microtrichial comb on tergite VIII not well developed (Fig 5c., 5d.)......... Frankliniella schultzei
Credit: JD Cluever, drawing by Jane Medley
Credit: JD Cluever
Credit: JD Cluever, drawing by Jane Medley
Credit: JD Cluever
Credit: JD Cluever
Control
Many methods of control exist for thrips including cultural, biological, and chemical. Cultural controls include using UV-reflective mulch and reducing the amount of nitrogen applied to the crop (Stavisky et al. 2002). UV-reflective mulch has been successfully used in tomato for reduction of thrips that vector plant disease (Funderburk et al. 2013).
Biological control includes the use of predatory mites and insects to reduce the population of pest thrips. Agents such as minute pirate bugs (Orius insidiosus) and predatory mites (Amblyseius swirskii) can be useful for controlling thrips populations and are commercially available (Arthurs et al. 2009).
Naturally occurring thrips predators in Florida strawberry fields include minute pirate bugs, ladybird beetles, lacewings, and predatory mites. Insecticide application decisions for thrips management in strawberry should take into account the presence of naturally occurring natural enemies and the species of thrips present.
Chemical control is another option available for the management of thrips populations. Thrips can often be difficult to control effectively with insecticides because their cryptic behavior limits their exposure to contact insecticides (Hansen et al., 2003). Flower thrips may be protected from systemic insecticides as there may be a lack of uptake into floral tissues (Cloyd and Sadof 1998). Thrips populations also display a great ability to build up resistance to insecticides (Jensen 2000). Resistance of Frankliniella occidentalis has been reported to insecticides in several chemical groups including the organochlorines, carbamates, organophosphates, neonicotinoids, pyrethroids, avermectins, spinosyns, and the phenylpyrazoles (Race 1961; Imamaraju et al. 1992; Jensen 2000; Morishita, 2001; Herron and James 2005; Weiss et al 2009; Kay and Heron 2010; Chen et al. 2011). When using chemical control, it is necessary to rotate insecticide groups to prevent a buildup of resistance in thrips populations. The application of broad spectrum insecticides may upset control by natural enemies. Biological control programs that integrate the use of insecticides compatible with the conservation of natural populations of minute pirate bugs have been implemented for fruiting vegetables in Florida (Funderburk et al. 2011). Broad spectrum insecticides may also cause western flower thrips populations to increase due to a reduction in the competition from native flower thrips (Paini et al. 2008).
References
Arthurs, S., C.L. McKenzie, J. Chen, M. Dogramaci, M. Brennan. 2009. Evaluation of Neoseiulus cucumeris and Amblyseius swirksii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biological Control. 49(1): 91-96.
Avila, Y., J. Stavisky, S. Hague, J. Funderburk, S. Reitz, and T. Momol. 2006. Evaluation of Frankliniella bispinosa (Thysanoptera: Thripidae) as a vector of the Tomato spotted wilt virus in Pepper. Florida Entomologist. 89(2): 204-207.
Bi-Song, Yue. 2001. Growth, Reproduction and field population dynamics of Frankliniella bispinosa (Thysanoptera: Thripidae). Entomologia Sinica. 8(3): 265-270.
Bottenberg, H., G. Frantz, and C. Mellinger. 1999. Refuge and cover crop plantings for beneficial insect habitats. Proceedings of the Florida State Horticultural Society. 112: 339-341.
Brodbeck, B.V., J. Stavisky, J.E. Funderburk, P.C. Andersen, and S. M. Olson. 2001. Flower nitrogen status and populations of Frankliniella occidentalis feeding on Lycopersicon esculentum. Entomologia Experimentalis et Applicata. 99(2): 165-172.
Chen, X., L. Yuan, Y. Du, Y. Zhang, J. Wang. 2011. Cross-resistance and biochemical mechanisms of abamectin resistance in the western flower thrips, Frankliniella occidentalis. Pesticide Biochemistry and Physiology. 101(1): 34-38.
Cloyd, R.A. and C.S. Sadof. 1998. Flower quality, flower number, and western flower thrips density on transvaal daisy treated with granular insecticides. HortTechnology. 8(4): 567-570.
Coll. M., I. Shouster, and S. Steinberg. 2005. Removal of a predatory bug from a biological control package facilitated an augmentative program in Israeli strawberry, pp. 501-509. In Second International Symposium on Biological Control of Arthropods, 12-16 September 2005, Davos, Switzerland. United States Department of Agriculture, Forest Service, Washington, USA.
Eger, J.E. Jr., J. Stavisky, and J.E. Funderburk. 1998. Comparative toxicity of spinosad to Frankliniella spp. (Thysanoptera: Thripidae), with notes on a bioassay technique. The Florida Entomologist. 81(4): 547-551.
Frantz, G. and T.R. Fasulo. 2013. Thrips KnowledgeBase. https://www.gladescropcare.com/thrips/pgspc.html
Funderburk, J. S. Reitz, P. Stansly, S. Olson, D. Sui, G. McAvoy, A. Whidden, O. Demirozer, G. Nuessly, and N. Leppla. 2011. Managing thrips in pepper and eggplant. https://edis.ifas.ufl.edu/publication/in401
Funderburk, J., S. Reitz, S. Olson, P. Stansly, H. Smith, G. McAvoy, O. Demirozer, C. Snodgrass, M. Paret, and N. Leppla. 2013. Managing thrips and Tospoviruses in Tomato. https://edis.ifas.ufl.edu/publication/in895
Gilstrap, F.E. and E.R. Oatman. 1976. The bionomics of Scolothrips sexmaculatus (Pergande) (Thysanoptera: Thripidae), an insect predator of spider mites. Hilgardia, Journal of Agricultural Science Published by The California Agricultural Experiment Station. 44(2): 27-59.
Hansen, E.A., J.E. Funderburk, S.R. Reitz, S. Ramachandran, J.E. Eger, and H. McAuslane. 2003. Within-plant distribution of Frankliniella species (Thysanoptera: Thripidae) and Orius insidiosus (Heteroptera: Anthocoridae) in field pepper. Environmental Entomology. 32(5): 1035-1044.
Herron, G.A. and T.M. James. 2005. Monitoring resistance in Australian Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) detects fipronil and spinosad resistance. Anustrailian Journal of Entomology. 44: 299-303.
Hoddle MS, Mound LA, Paris DL. 2012. Thrips of California. CBIT Publishing, Queensland.
Hunter, W.B. and Ullman. 1989. Analysis of mouthpart movements during feeding of Frankliniella occidentalis (Pergande) and F. schultzei Trybom (Thysanoptera: Thripidae). International Journal of Insect Morphology and Embryology. 18(2/3): 161-171.
Immaraju, J.A., T.D. Paine, J.A. Bethke, K.L. Robb, and J.P. Newman. 1992. Western flower thrips (Thysanoptera: Thripidae) resistance to insecticides in coastal California greenhouses. Journal of Economic Entomology. 85(1): 9-14.
Jensen, S. 2000. Insecticide resistance in western flower thrips, Frankliniella occidentalis. Integrated Pest Management Reviews. 5(2): 131-146.
Kay, I.R. and G.A. Herron. 2010. Evaluation of existing and new insecticides including spirotetramat and pyridalyl to control Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) on peppers in Queensland. Australian Journal of Entomology. 49(2): 175-181.
Kirk, W.D.J., and L.I. Terry. 2003. The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agricultural and Forest Entomology. 5(4): 301-310.
Kumm, S. and G. Moritz. 2009. Life-cycle variation, including female production by virgin females in Frankliniella occidentalis (Thysanoptera: Thripidae). Journal of Applied Entomology. 134(6): 491-497.
Lewis, T. 1991b. Feeding, Flight and dispersal in thrips. pp. 63-70. In Conference: Toward understanding thysanoptera. B.L. Parker, M. Skinner, L. Trevor. (eds.) Burlington, VT. General Tech. Rep. NE-147, Randall, PA: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. https://www.fs.usda.gov/research/treesearch/4216
Martin, K.W., A.C. Hodges, and N.C. Leppla. 2012. Citrus pests Florida flower thrips. http://idtools.org/id/citrus/pests/factsheet.php?name=Florida+flower+thrips
McDonald, J.R., J.S. Bale, and F.A. Walters. 1998. Effect of temperature on development of the western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). European Journal of Entomology. 95:301-306.
Milne, J.R., G.H. Walter, and G.C. Sabio. 1996. The importance of non-pollen parts as food sources for the common blossom thrips, Frankliniella schultzei. Entomologia Experimentalis et Applicata. 78: 271-281.
Milne, M. and G.H. Walter. 1997. The significance of prey in the diet of the phytophagous thrips, Frankliniella schultzei. Ecological entomology. 22: 74-81.
Morishita, M. 2001. Toxicity of some insecticides to larvae of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) evaluated by the petri dish-spraying tower method. Applied Entomology and Zoology. 36(1): 137-141.
Morse, J.G. and M.S. Hoddle. 2006. Invasion biology of thrips. Annual Reviews of Entomology. 51: 67-89.
Mossler, M.A. 2013. Florida crop/pest management profiles: strawberry. https://doi.org/10.32473/edis-pi042-2013
New Brunswick. 2014. Agriculture, Aquaculture, and Fisheries, Fruit Bronzing in Strawberry. https://www2.gnb.ca/content/gnb/en/departments/10/agriculture/content/crops/small_fruits/fruit_bronzing.html
Nielsen, M.C., D.A.J. Teulon, R.B. Chapman, R.C. Butler, G.M. Drayton, and H. Philipsen. 2010. Comparison of life history parameters of two Frankliniella occidentalis (Thysanoptera: Thripidae) strains in New Zealand. Environmental Entomology. 39(2): 303-311.
Paini, D.R., J.E. Funderburk, and S.R. Reitz. 2008. Competitive exclusion of a worldwide invasive pest by a native. Quantifying competition between two phytophagous insects on two host plant species. Journal of Animal Ecology. 77(1): 184-190.
Pinent, S.M.J., A. Nondillo, M. Botton, L.R. Redaelli, C.E. da C. Pinent. 2011. Species of thrips (Insecta, Thysanoptera) in two strawberry production systems in Rio Grande do Sul State, Brazil. Revista Brasileira de Entomologia. 55(3): 419-423.
Polito, V.S., K.D. Larson, and K. Pinney. 2002. Anatomical and histochemical factors associated with bronzing development in strawberry fruit. Journal of American Society of Horticultural Science. 127(3): 355-357.
Race, S.R. Early-season thrips control on cotton in New Mexico. Journal of Economic Entomology. 54(5): 974-976.
Rhodes, E. M., O. E. Liburd, and G. K. England. 2012. Effects of southern highbush blueberry cultivar and treatment threshold on flower thrips populations. Journal of Economic Entomology 105: 480-489.
Stavisky, J., J. Funderburk, B.V. Broadbeck, S.M. Olson, and P.C. Andersen. 2002. Population dynamics of Frankliniella spp. and tomato spotted wilt incidence as influenced by cultural management tactics in tomato. Journal of Economic Entomology. 95(6): 1216-1221.
Steiner, M.Y. and S. Goodwin. 2005. Management of thrips (Thysanoptera: Thripidae) in Australian strawberry crops: within-plant distribution characteristics and action thresholds. Australian Journal of Entomology. 44(2): 175-185
Weiss, A., J.E. Dripps, and J. Funderburk. 2009. Assessment of implementation and sustainability of integrated pest management programs. Florida Entomologist. 92(1): 24-28.
Williams, R.N. 1998. Flower Thrips. In J.L. Maas (ed.) pp. 91. Compendium of Strawberry diseases. 2nd edition American Phytopathological society. St. Paul, Mn.
Zalom, F.G., M.P. Bolda, P.A. Phillips, and N.C, Toscano. 2014. UC Pest Management Guidelines: Strawberry, Western Flower Thrips, Scientific Name: Frankliniella occidentalis. UC ANR Publication 3468. https://ipm.ucanr.edu/agriculture/strawberry/western-flower-thrips/#DAMAGE