
This publication is part of SL253, Nutrition of Florida Citrus Trees, 3rd Edition. For references, a glossary, and appendices, please refer to the full document at https://edis.ifas.ufl.edu/ss478.
Essential Nutrients
Seventeen elements are essential for the growth and functioning of green plants. Carbon (C), hydrogen (H), and oxygen (O), which make up about 95% of tree biomass, are provided by nature. C and O are taken up by leaves as carbon dioxide (CO2) from the air, and they combine with H, taken up as water by the roots, to produce carbohydrates. Photosynthesis takes place in chlorophyll-bearing cells, using light as an energy source. Carbohydrates, together with proteins, fats, and other organic compounds derived from them, are the true plant foods. They are used to make new plant tissues and provide energy for growth and fruiting.
The other 14 mineral elements are nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), iron (Fe), zinc (Zn), manganese (Mn), boron (B), copper (Cu), molybdenum (Mo), chlorine (Cl), and nickel (Ni). Florida's sandy soils often do not contain a sufficient supply of these nutrients, so growers may need to provide them through fertilizer application.
When any essential element is in short supply, tree function is restricted. A severe shortage of an element typically produces a characteristic deficiency symptom exhibited by the leaves, which usually persists until the deficiency is corrected. Sometimes twigs and fruits may also exhibit characteristic symptoms. Sometimes two or three elements are deficient in varying degrees, resulting in confusing visual symptoms. Conversely, excessive amounts of some elements may be present in the soil and may prevent the tree from functioning properly. Visual symptoms and leaf and soil analysis are all useful to evaluate nutritional status.
Mineral nutrients are divided into macronutrients, which are elements that plants require in large amounts (N, P, K, Ca, Mg, S), and micronutrients, which are needed only in small amounts (Fe, Zn, Mn, B, Cu, Mo, Ni, Cl) (Table 1). The macronutrients are divided into two groups, primary elements (N, P, and K) and secondary elements (Ca, Mg, and S). Micronutrients are sometimes referred to as "minor" or "trace" elements, but these terms are misleading. For example, the role of Fe in plant metabolism should not be considered less important than the role of K. Iron deficiency can result in total crop loss, so its role is not a "minor" one, and it is not of minor importance. The difference between Fe and K is in the amount required by plants, so the use of the terms micro- and macronutrients is more appropriate.
Relative essential mineral element composition of a 6-year-old 'Hamlin' orange tree (excluding Cl and Ni). (Derived from Mattos et al. 2003).
Macronutrients and Citrus Production
Nitrogen (N) is of primary importance in citrus production. It has more influence on tree growth, appearance, fruit production, and fruit quality than any other element. When N is in short supply, growth is limited and the foliage becomes pale green or yellowish in color. When N is supplied to bearing trees at sub-optimal rates for a long period of time, the trees adjust by recycling N from the oldest leaves into the new ones, and the old leaves are shed prematurely, leading to a thin canopy. Inside leaves that should function for up to 2 years or more are reduced to a life of 1 year or less. The green color of the remaining leaves may be nearly normal, but the canopy is hollow inside. Yield can be reduced somewhat, but the typical yield response curve (Figure 2) shows that a rather large decrease in N supply is required before yield is greatly decreased.
In cases of persistent N shortage, defoliation, fruit drop, and shoot death can occur. When N is present above visible deficiency, shoot growth and yield increase with increasing N supply up to an optimum N rate. A sufficient N concentration in the tree is required for maximum vegetative growth, flowering, and fruit yield. A high N concentration increases tree growth and may require increased applications of other elements, particularly K. Luxury consumption of N can lead to excessive vegetative growth at the expense of yield.
Phosphorus (P) is listed on a fertilizer label as P2O5, referred to as available phosphoric acid. Most sandy soils used to produce citrus contain sufficient residual P that accumulated from previous fertilizer applications. Phosphorus does not readily leach if the soil pH is 6.0 or higher, and the fruit crop removes very little (Table 3). Therefore, regular P fertilizer application is usually not necessary. Some previously noncultivated soils used for new citrus plantings are naturally low in P, so fertilizer P may be needed for several years.
Potassium (K) (also called potash) is listed on a fertilizer label as K2O, and is important to yield, fruit size, and juice quality. Potassium does not accumulate to a great extent in sandy soils used to produce citrus, even with repeated fertilizer applications. Potassium deficiency is not common but may develop with high N rates and high fruit production. Too little K can slow vegetative growth and result in thinning of the topmost foliage. A deficiency reduces fruit number and size, increases fruit creasing, plugging, and drop, and decreases juice soluble solids, acid, and vitamin C content. A high K fertilizer rate does not increase cold hardiness of citrus trees.
Calcium (Ca) is the most abundant element by weight in citrus trees, residing mainly in the leaves. Ca is rarely deficient in a tree because occasional applications of CaCO3 (lime) are used to control soil acidity and because Ca is present in irrigation water. Florida's alkaline soils have an abundance of Ca because they contain free limestone.
Magnesium (Mg) is needed to produce chlorophyll. A deficiency produces a characteristic chlorotic pattern and may cause premature defoliation. Seedy varieties may need more Mg than seedless ones because seeds store a large amount of Mg. Dolomitic limestone is often used to correct acidity and supplies slowly available Mg. Calcium is abundant in alkaline soils, which can be antagonistic to Mg uptake.
Sulfur (S) is utilized by citrus trees in an amount similar to P uptake. It is supplied with fertilizers including ammonium sulfate and sulfates of micronutrient metals. Sulfur is a major component of the soil organic fraction and becomes available to plants as organic matter decomposes. Sulfur is also present in some irrigation water sources. When S is deficient in a citrus tree, the symptom looks like N deficiency.
Micronutrients and Citrus Production
Iron (Fe) deficiency causes a chlorotic pattern that first appears on young shoots. It occurs in trees growing in alkaline soil, waterlogged soil, or very low organic matter soil (sand ponds). Other Fe deficiency problems have occurred where Cu is high in the soil.
Copper (Cu) deficiency causes conditions known as fruit corking, ammoniation, and dieback, which can be corrected by applying Cu fertilizer to the soil. Copper should not be included in fertilizer if foliar Cu sprays are used, or if a grove soil test shows sufficient Cu (see Chapter 4). For new plantings on previously noncultivated Flatwoods soils, Cu should be included in the fertilizer.
Zinc (Zn) deficiency symptoms are expressed in citrus trees as severe chlorosis where leaf tissue becomes nearly white, except for green veins. New leaves grow progressively smaller as the deficiency becomes more severe, and shoot internodes become shorter, causing a rosette effect. Severe Zn deficiency restricts growth and reduces fruit yield.
Manganese (Mn) deficiency produces a mild form of chlorosis on acidic, sandy soils. The "marl chlorosis" found on calcareous soils is the result of combined deficiencies of Mn and Zn, and sometimes Fe. A temporary mild deficiency pattern on new shoots is not detrimental to growth or fruiting of citrus trees. Corrective measures should only be taken in the case of persistent deficiency symptoms.
Boron (B) deficiency causes excessive fruit drop, gum formation on the outside of the fruit, and brown areas in the albedo and central axis. It sometimes occurs when growers use only high-analysis fertilizers (without micronutrients), or following a prolonged drought. Boron should be applied every year either as a soil or foliar application but not both.
Molybdenum (Mo) deficiency produces a symptom described as "yellow spot." Unlike other nutrients, Mo is less available in acidic soils than in slightly alkaline soils. Mo deficiency is rare in Florida. If it occurs, the soil usually has become undesirably acidic with time. Liming the soil is effective in relieving the deficiency.
Supplying Nutrients to Citrus Trees
A sufficient supply of essential nutrients is critical to nutrient management and sustainability. If a single element is below the critical availability level, crop growth and yield will fall even if the other elements are in sufficient supply. A balance of available nutrients is a key component to profitability because it allows for positive nutrient interaction. For example, in the case of N fertilization, a shortage of another nutrient could decrease N uptake, reduce N use efficiency, and increase the potential for N loss.
Soil application of macronutrients is favored over foliar application due to the high uptake demand by citrus trees. However, fertilizer applied to the soil is subject to various fates including leaching, runoff, and fixation to forms not available to plants. Solution fertilizers applied to the tree foliage are less prone to these losses, but only small quantities of nutrients can enter leaves. Foliar fertilizer application may be considered for nutrients including N, P, K, Mg, Zn, Mn, and B. It is especially useful when soil properties like high pH inhibit nutrient availability.
Foliar fertilizer application can reduce or eliminate soil applications of micronutrients since they are required in low amounts (Table 1). Foliar application is the fastest method of getting nutrients into plants over the short term when a nutritional deficiency is diagnosed, but it should not be relied upon for long-term tree nutrition unless the soil is calcareous (see Chapter 11).
Fertilization represents a relatively small percentage of the total cost of citrus production, but it has a large effect on potential profitability. Visual evaluation of nutritional status, soil and plant analysis, field history, production experience, and economics are all important guidelines to use when making fertilizer rate and source decisions.
Nutrient Behavior in Florida Soils
Plant nutrients exist in both organic and inorganic forms in soil. Organic forms are found in fresh plant residue, soil organic matter (humus), living soil organisms (e.g., bacteria and fungi), soil amendments (e.g., biosolids or compost), and synthetic organic materials (e.g., some N fertilizers). Organic materials are the key component of nutrient recycling. They are a stable storehouse of plant nutrients because they are not readily lost from the soil.
Nutrients associated with organic matter are not immediately plant-available, but are slowly released as the material is decomposed by soil microbes. The decomposition rate depends on the material's physical and chemical characteristics and the climate. Florida's warm and humid conditions are ideal for decomposition of almost any organic material, so organic matter does not typically accumulate in citrus grove soils. Nutrients are continuously released in inorganic form as decomposition proceeds. The recycling process is complete once these nutrients are taken up by growing plants. Many of the nutrients in citrus tree residues (dropped leaves, twigs, and fruit; dead roots) are returned to the tree in this manner.
Inorganic plant nutrients exist in solid form (minerals or precipitates), in adsorbed form (bonded to a solid phase material), on the cation exchange complex (Chapter 2, Figure 5), or in the soil solution. The ionic nutrient forms that plants use (Table 2) must dissolve, desorb, or exchange into the soil solution before they can be taken up. If the soil solution is not replenished with nutrients rapidly enough to satisfy plant demand, plant nutrition will be less than optimum.
In an intensive crop production system, fertilizers added to the soil supplement the natural nutrient supply and prevent nutrient deficiencies. Most fertilizers applied to citrus trees are inorganic minerals or soluble salts that quickly dissolve into plant-available (ionic) form. The soil can react with some of these ionic forms, rendering them unavailable to plants. In the absence of these reactions, nutrients may leach with water that percolates through the root zone. The general characteristics and behavior of nutrients in sandy Florida soils planted to citrus are outlined below.
Nitrogen
- 95% of the natural N that resides in the soil is associated with organic matter. Soil humus contains about 5% N. The N release from organic matter depends on how much is there and how fast the material decomposes. This release rate is fast enough to support plant growth in a natural landscape, but it is too slow for intensive agricultural production on sandy soils.
- Biological ammonification converts organic N to mineral N (NH4+). Ammonium is also a component of some mineral N fertilizers. Nitrification, which also depends on microbial activity, converts NH4+ to NO3- in days to weeks. Thus, soil solution N is dominated by nitrate, which is negatively charged. There is no mechanism to hold nitrate in the soil, so it leaches easily.
- Most of the N lost from soils is a result of N loading of the soil from fertilizer or animal waste application, followed by N leaching from the soil with excessive rainfall or irrigation.
Phosphorus
- P occurs naturally in some Florida soils as calcium phosphate minerals. These minerals can also slowly form following P fertilizer application. Soil phosphates are relatively insoluble, which can affect plant availability.
- If a soil has the capacity to adsorb, or "fix" P, then added P will accumulate in the root zone. Phosphorus fixation occurs when soluble P forms nearly insoluble compounds with Fe or aluminum (Al) at low pH, or Ca at high pH. The best P availability in these soils occurs around a pH of 6.5.
- Florida's sandy soils may or may not have the capacity to hold applied P fertilizer, depending on the type of sand present. Sand coated with Fe or Al compounds can fix P in the root zone, while noncoated sand cannot (Chapter 2, Figure 6). If a soil is dominated by noncoated sand, P may leach.
- Adsorbed P can be transported via surface runoff (erosion), while soluble P can be transported via leaching. Phosphorus loss from the soil results from long-term loading of the soil with P from animal wastes or fertilizers, followed by erosion of soil and organic matter particles or leaching, depending on the soil.
Potassium
- Florida soils are naturally low in K, so intensive agricultural production requires the use of K fertilizer.
- The ionic form of K can be held by the soil cation exchange complex (Chapter 2, Figure 5), which delays leaching. However, Florida soils planted to citrus have naturally low cation exchange capacity in the root zone (Appendix A, Table 26), so K+ leaches almost as readily as NO3-.
- K is not fixed in sandy soils and does not form insoluble compounds, so it is easily lost from the root zone. Thus, K fertilizer application is required every year in Florida citrus groves.
Calcium and Magnesium
- Ca and Mg exist as solid compounds in the soil (mostly in combination with carbonate or phosphate) and in ionic forms held by the cation exchange complex.
- Solid forms of Ca and Mg are sparingly soluble and can reside in the soil for many years if the pH is not too acidic. Dissolution is more rapid at low pH, which is the basis of the liming reaction.
- Because they are divalent cations, Ca and Mg dominate on the cation exchange complex, which limits their mobility in soil.
Sulfur
- 90% of the S that occurs naturally in soils is associated with organic matter. Soil humus contains about 0.5% S. Like N release, S release from organic matter depends on quantity and decomposition rate. Organic S release combined with S from other sources like rain or irrigation water usually provides this nutrient to plants fast enough even in intensive agricultural production.
- The plant-available form of sulfur (sulfate) is a negative ion, which makes it prone to leaching. Sulfate can be adsorbed by soils, but this reaction is stronger deeper in the soil profile than the main root zone.
- Calcium sulfate (gypsum) is a sparingly soluble compound that is applied as a long-term source of available Ca, but it also supplies S to plants.
Copper, Iron, Manganese, and Zinc
- These micronutrients form compounds that are only slightly soluble in sandy soils; thus, they are not mobile nutrients. Solubility increases somewhat as pH decreases (Figure 1), so it is important to not overlime a soil. At alkaline pH, some plants suffer micronutrient deficiencies due to almost total insolubility.
- If applied to the soil as soluble fertilizer, these micronutrients will precipitate near the soil surface.
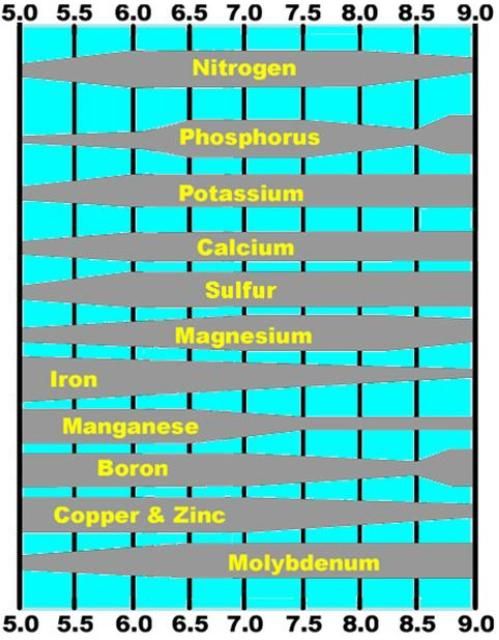
Boron
- The plant-available form of B is negatively charged (borate), so it can easily leach from sandy soil.
- B needs to be applied regularly in Florida citrus groves, but there is a narrow range between deficiency and toxicity.
Molybdenum
- Mo is the only micronutrient that increases solubility as soil pH increases. Thus, it is an immobile nutrient within the soil pH range that is favored for citrus tree growth.
Citrus Nutrient Requirements
This section describes the typical citrus yield response curve and discusses nutrient requirements in relation to anticipated yield. Fertilizer rate guidelines for nonbearing and bearing trees are provided in Chapter 8.
The relationship between nutrient supply and yield of a wide variety of annual and perennial crops has been studied for decades. The relationship between plant response (yield) and fertilizer rate is called the yield response curve. The shape of this curve is similar for a range of crops and conditions.
The curves in Figure 2 illustrate how citrus yield increases with increasing N rate for two conditions. Fertilizer N is used in this example, but the nature of the response curve is similar for other limiting nutrients. At very low N rates there is a large yield response to each added unit of N. As yield increases, each additional unit of N results in a smaller increment in yield. This smaller response to increasing input is also referred to as the law of diminishing returns. The two response curves in Figure 2 compare the effect of N rate when other factors are not limiting and the response when yield is limited to one-half by a second factor. The shapes of the curves are similar, and the rate of N where the slope levels off is only slightly higher for the more productive grove.
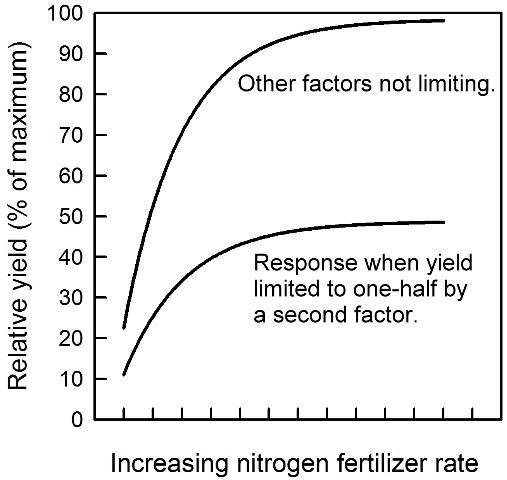
The yield response curves shown never completely flatten out, indicating that ever higher N rates theoretically will produce small additional yield increases. Because fertilizer cost has traditionally been a small portion of total production cost, high N rates were commonly applied to produce the highest possible yield. However, experiments with citrus have rarely demonstrated a benefit of N fertilizer rates higher than about 200 lb/acre, regardless of the production potential. Instead, yield declined in several experiments when N rate was increased beyond the optimum range.
Nutrients removed with the harvested crop must be replaced. The amount the crop removes varies from a fraction of a lb/acre for some of the micronutrients to as much as 100 lb/acre of N or K from a high-producing grove (Table 3). For oranges, approximately 0.12 lb N/box is removed with the harvest. Therefore, crop removal ranges from 12 lb N/acre for a 100 box/acre yield to around 100 lb N/acre for a grove producing 800 boxes/acre.
Nutrient uptake from applied fertilizers is not 100% efficient, so more nutrients must be applied than the minimum required by the tree. N use efficiency, expressed as lb N removed by the crop divided by lb N applied, ranges from 0.2 to 0.4 in groves with low to moderate yield. However, N efficiencies around 0.5 have been observed in groves with a good production record. Application of 200 lb N/acre supplies sufficient N for an 800 box/acre orange yield when N use efficiency is 0.5. A grower using the latest fertilization technology (e.g., fertigation, controlled-release fertilizers) with good irrigation management may be able to exceed an N use efficiency of 0.5.
Nutrition and Fruit Quality
Florida has the highest citrus fruit quality standards in the world. The most important quality factors for Florida citrus growers, production managers, processors, and packers include fruit juice content, soluble solids and acid concentrations, soluble solids/acid ratio, fruit size, and color. Florida citrus growers discern between quality factors for the fresh and processing markets. For example, fruit size, shape, color, and maturity date are most important for fresh fruit, but high juice content and soluble solids are desired for processed fruit. Fruit quality is affected by factors including cultivar, rootstock, climate, soil, pests, irrigation, and nutrition.
The effects of nutrition and irrigation on fruit quality should be understood and taken into consideration by citrus growers to increase profitability, enhance sustainability, and improve worldwide competitiveness. Excessive irrigation and fertilization reduce fruit quality, so supplying sufficient nutrition and using sound irrigation scheduling techniques should be high priorities of every grower. Citrus trees require a properly designed, operated, and maintained water management system and a balanced nutrition program formulated to provide specific needs for tree maintenance and expected yield and fruit quality.
Irrigation is a major component of fertilizer program efficiency. Citrus trees with sufficient water and nutrients grow stronger, tolerate pests and stresses better, yield more consistently, and produce good-quality fruit. On the other hand, excessive or deficient irrigation or fertilization may result in poor fruit quality.
The most important management practices influencing fruit quality are irrigation and N, P, K, and Mg nutrition. Some micronutrients like B and Cu can also affect fruit quality, but only if they are deficient. In general, when any nutrient element is severely deficient, fruit yield and fruit quality will be negatively affected.
Trends in fruit quality response to increasing nutrient and water availability are described and summarized below:
Nitrogen
- Increases juice volume and color, total soluble solids (TSS), and acid concentration.
- Increases TSS per box and per acre. However, excessive N, particularly with inadequate irrigation, can result in lower yields with lower TSS per acre.
- Decreases fruit size, weight, and peel thickness.
- Increases green fruit at harvest. High N may delay color break and increase re-greening of Valencia oranges
- Increases creasing and scab, but decreases peel blemishes like wind scar, mite russeting, and rind plugging.
- Reduces stem-end rot and green mold of fruit in storage.
Phosphorus
- Reduces acid concentration, which increases TSS/acid ratio.
- Increases number of green fruit.
- Reduces peel thickness.
- Increases expression of wind scar but reduces that of russeted fruit.
Potassium
- Decreases juice content, TSS, TSS/acid ratio, and juice color.
- Increases acid content.
- Increases fruit size, weight, green fruit, and peel thickness.
- Reduces splitting, creasing, and fruit plugging.
- Reduces stem-end rot of fruit in storage.
Magnesium
- Slightly increases TSS/box and TSS/acid ratio.
- Slightly increases fruit size and weight.
- Decreases rind thickness.
Irrigation
- Increases juice content and TSS/acid ratio.
- Reduces TSS and acid concentration.
- Increases fruit size and weight and green fruit at harvest
- Decreases rind thickness.
- Increases blemish from wind scar, scab, and Alternaria brown spot, but reduces rind plugging.
- Reduces stem-end rot, but increases green mold of fruit in storage.
Specific effects on juice and external fruit qualities summarized in Table 4 are based on numerous field experiments conducted over many years that evaluated the response of oranges to irrigation and fertilization practices. Most of these effects were consistently observed, but some of them appeared to depend on local conditions and growing regions. These observations are useful to help develop a strategy aimed at improving fruit quality for a particular variety or location.
Grove Management Practices
Management practices that improve fertilizer nutrient-use efficiency include:
- Using a leaf and soil testing program (Chapter 4).
- Selecting fertilizer materials, ratios, and blends that match nutrient requirements (Chapters 6 and 8).
- Carefully placing fertilizer over the root zone (Chapter 7).
- Timing to avoid the rainy season (Chapters 8 and 10).
- Split applications (Chapter 8).
- Irrigation management to maximize production and minimize leaching (Chapter 9).
- Applying N fertilizer at a rate consistent with historical or expected production (Chapter 8).
Groves with non-nutritional limiting factors do not produce more fruit if the grower exceeds basic fertilizer requirements in an attempt to boost yield. Instead, excess fertilizer is not used by the tree, efficiency declines, and potential for leaching loss increases.
Interactions of Nutrition with Other Grove Practices
Nutrition management interacts with irrigation, pest control, weed management, and vegetative growth control (hedging and topping). Nutrition and irrigation are linked through fertigation and the need to provide maximum nutrient uptake while minimizing nutrient leaching. Water and nutrient uptake efficiency increases as trees mature due to greater interception by closely interwoven root systems. Fertilization and irrigation outside the root zone is economically and environmentally unsound and promotes weed growth.
Nutrient Considerations for Disease and Insect Control
Luxuriant growth caused by excessive fertilization or irrigation may increase incidence of foliar and blossom fungal diseases like scab, Alternaria brown spot, and postbloom fruit drop (PFD). Excessive vegetative growth may also increase insect pest problems including the citrus leafminer and the Asian citrus psyllid, which vectors huanglongbing (citrus greening) disease. Controlling tree growth at containment size through pruning is more difficult when vegetative growth is promoted by excessive inputs. Such excess vegetative growth competes with fruit production and may suppress it.
Until specific fertilization recommendations for groves infected with citrus greening disease are developed, groves should be provided with sufficient nutrition to maintain current fruit production (see Chapter 8). However, if a grove is being visually monitored for greening symptoms, it is important to
minimize signs of Zn deficiency so the disease can be more easily detected. Likewise, tree growth (particularly of young trees) during the fall and winter makes it difficult to control psyllids, so fertilizer application during this period is discouraged.